
-----
Want gold colored finishes without use of gold
Q. Hello Sir,
We produce copper statues . I would like to know if there's any process or any chemical that is used to coat the copper into gold colour without using gold. I just want to get the golden looks on it.
- Kathmandu Bagmati
October 26, 2023
A. Hi Sunhill.
How to make something look like it contains more gold that it actually does has been a common question for millennia. Surface enrichment of low carat gold and gilding were early answers. Gold electroplating was a great solution because it made something look identical to real solid gold by putting the real gold only on the surface.
As gold plating technology improved, and as people became satisfied with shorter lifetimes for the articles, the plated layer got thinner & thinner, these days sometimes being a fraction of a micron, but clear coated for durability.
But even a fraction of a micron of gold is too much for some applications and the question arises of making something look like gold while containing zero gold. Part of the answer is the saying that "only gold looks like gold", but "how close does it look to gold" is important.
If the volume of statues justifies it, titanium nitride PVD coating looks very much like gold, but the million dollar machine it requires puts it out of the range of small shops. There are various electroplated brass formulations mentioned on this page and on thread 32087, thread 51788, and thread 57394 which are probably your best bet.
I've never seen a paint that could come close to fooling even me, and I'm no jeweler -- so I don't think paint is the way forward.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Good day,
Anodizing titanium at a particular voltage can give a close-by gold-like color.
Success, Peter
- Leuven, Belgium
January 12, 2024
A. If you use 20 gm citric acid /1 lit water electrolyte at 25 °C temperature 42,5 - 47,7 V ...
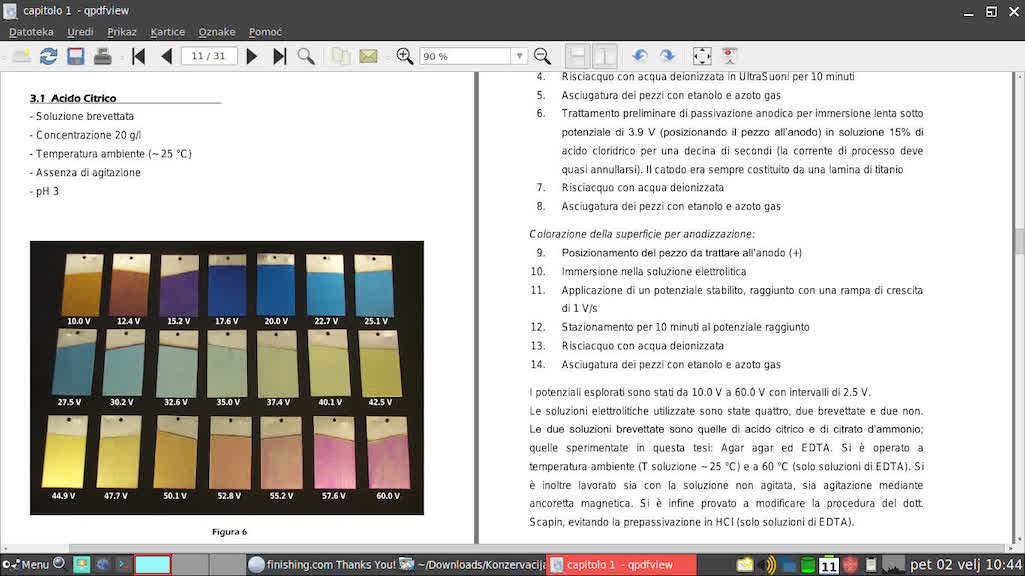
Hope it helps and good luck!
Goran Budija- Cerovski vrh Croatia
February 2, 2024
A. As far as I know casting copper is not an easy task, probably you use brass or bronze ...
Brass can be gold coloured with thiosulphate-based patina : sodium thiosulphate 240 gms/copper acetate 25 gms/ citric acid 30 gms/ 1 lit water. Pale gold 60 minutes, gold 80 minutes immersion (according to Fishlock,D. Metal Colouring,Teddington 1963, p.219) Hope it helps and good luck!
- Cerovski vrh Croatia
February 3, 2024
⇩ Related postings, oldest first ⇩
Q. Dear Sir,
I am trying to find some solution or formula that can give me the gold color for decorative purposes without the use of gold in electroplating process
I have heard about some alloys that contain copper & tin that would give me some great result.
thank you in advance for your help.
plating shop - Damascus, Syria
2005
A. Hi There,
It's true that you can get a yellowish colour for a top coat which is called yellow bronze - alloy of Copper 85 % and Tin 15 % but the finish may not be as similar as gold finish and also the tarnishing tendancy is very high; it's major application is for nickel free process.
The best works out for you is electrophoretic gold colour process which is very popular these days ... with a good gold and many more colours, and durability ... cha cha cha

Praveen Kumar
plating process supplier
Mumbai, India
A. The closest and cheapest alloy which looks like gold is brass (Copper-zinc).
You can find some good and free formulations in the Metal Finishing directory book.
If you have any difficulties to find the directory, I can send you one (give me an address in Europe or USA as there is no way I can send it directly to you ... but with time, I am sure we will be able to send books to each other).
Best regards

Sara Michaeli
Tel-Aviv-Yafo, Israel
A. Try either brass plating, lacquers, or titanium nitride by vacuum deposition.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
? Hi Shafeek Saati
Can you tell me what is the product made of (Substrate) which you want to make look like gold.
In case you can make it out of aluminum extrusion, you could brighten the same and have it dyed in golden color. This would make it look exactly like Gold or Brass.
The method is fairly cheap and inexpensive. What is important though is the volume of product you must process and a good understanding of aluminum brightening, anodizing and coloring.
Regards,
- Gujarat, India
Electrophoretic lacquer
for Shops, Specifiers, & Engineers

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
Shafeek ...
No yellow bronze nor brass. It is electrophoretic gold dye lacquer you are looking for. This can be either acrylic or polyurethane lacquer with gold dye of 24 karat colour. The start up investment is going to be expensive because you will require an ultrafilter and that alone will cost you 200 US$. The water generally used is of less than 5 microsiemens. But the running cost is going to be 1/50th of gold plating.

T.K. Mohan
plating process supplier - Mumbai, India
2005
A. Electrophoretic gold is a good idea. But If I am correct, it is an anodic process and therefore it cannot be applied to all metals.

Sara Michaeli
Tel-Aviv-Yafo, Israel
Ed. note: We believe that in the early days of electropainting, it was usually anodic electrodeposition; but these days it's almost universally cathodic electrodeposition (CED)
Q. We received a response from T.K.Mohan Mumbai, India he has suggested electrophoretic gel dye lacquer and said it will be possible with ultrafilter which cost 200 US Dollars.
From where I can purchase this filter and the complete process.
Thank you and best regards.
jewellers - Lahore, Pakistan
2005
Ed. note: I think TK was suggesting that the capital cost of such a line is very high, but made a typo in the cost of the ultrafilter. He probably meant to say either that an ultrafilter is "in excess of $20,000 U.S." or that it can cost "as much as $200,000" :-)
Q. Someone know about electrophoretic gold plating? It is not gold, just the color is like gold. I would love to have some information. I would like to plate just the color of gold.
Karine Martimbianco- Fairfax, Virginia, USA
April 19, 2018
A. Hi Karine. As long as you don't expect too much in terms of actual resemblance to gold, electrocoating followed by a metallic gold colored post-dip could be what you are looking for.


(CED coating with gold colored post dye; same part, different lighting)
The above part was just a "one-off" coating on a zinc die-casting for a corrosion study which finishing.com did for the International Zinc Association. I'm confident that vendors could improve the consistency with a little development effort and practice on the particular parts, but I don't think very many people would believe it's actually gold :-)
Please see topic 49008 for more about electrophoretic lacquering, or search the site for e-coating, electropainting, CED, or electrophoretic lacquer (all of these terms are generally synonymous). Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Dear Sir (TK Mohan),
please go through if this is correct:
CN106634443A Formula and preparation process of electrophoretic paint
- Hyderabad
July 22, 2023
Ed. note: Sorry, we don't know what your posting means :-)
Are you trying to provide an answer for someone? More words please. Please introduce us to who you are, what you do, and tell us your situation so a reader can help you or profit from your help. Thanks!
Q. I want gold color plating on brass article but cannot afford real gold plating. Can someone help? Have tried buffing and then lacquering but doesn't look golden.
Tarun Jain- Mumbai, Maharashtra, India
February 26, 2014
A. Hi cousin Tarun. It sounds like you are looking for a gold toned lacquer that is better than the ones you've tried :-)
We discourage commercial replies like "Brand Y is better than Brand X" ( huh? why?) but G.J. Nikolas [a finishing.com supporting advertiser] is a lacquer specialist who may have the answer you are looking for.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. The only thing that truly looks like gold is real gold.
Chris Owen- Nevada, Missouri, USA
A. We have a PVD coating that we apply to brass parts which is a good match to 24 kt gold. We developed it for our faucets. Not only is it less expensive than gold plating, it doesn't wear off. Downside is that PVD is a batch process, so we don't like to take on small projects.

Jim Treglio - scwineryreview.com
PVD Consultant & Wine Lover
San Diego, California
A. Gold has always been expensive and many attempts have been made to find substitutes.
In 1720, James Pinchbeck introduced a material that was widely used and is still popular with antique collectors. Pinchbeck alloy is simply a modified brass containing 83% copper and 17% zinc. The colour is very close to 18 kt gold.
This is very similar to Dutch Metal copper 84%, Zinc 16% which is commonly used as a substitute for gold leaf gilding.
A more modern substitute is used for euro coins and is known as Nordic Gold Copper 87%, Aluminium 5%, Zinc 5%, Tin 1%
None of these is usually lacquered.

Geoff Smith
Hampshire, England
Q. Mr Geoff.
Nordic Gold is suitable in Stainless steel Surgical instruments? Can you explain in detail.
Thanks in Advance and waiting for reply.
instruments - Punjab, Pakistan
March 11, 2014
A. Hi Muhammed
Copper alloys are well documented as having anti-bacterial properties and I have scalpel holders which appear to be made of some type of light coloured bronze so you may have found a suitable application. However, I suspect that medical instruments are subject to regulation so I suggest that you check with end users before going ahead.

Geoff Smith
Hampshire, England
![]() Thanks for the pair of very informative answers, Geoff. I think I would add that an exact color match with gold should not be important to surgeons because the intent is certainly not to try to fool them :-)
Thanks for the pair of very informative answers, Geoff. I think I would add that an exact color match with gold should not be important to surgeons because the intent is certainly not to try to fool them :-)
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I have to know the steps of making nordic gold liquid solvent using cyanide, potassium and sodium chloride. Please anyone help me.
ravi kumara m rstudent - Bangalore Karnataka India
May 27, 2014
A. Hi Ravi. Please take a paragraph and introduce your situation because your question by itself isn't clear enough. Nordic gold is, as Geoff Smith has previously told us, and per the link we provided to Wikipedia, a copper alloy with a little zinc, aluminum, and tin in it, and no gold at all. So what do you mean by "liquid solvent"? And what does cyanide have to do with the situation? Sorry, but I'm lost.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Dear sir
I have to give gold color to silver, copper, brass jewellery without using gold. Please help me.
student - Bangalore Karnataka India
May 28, 2014
A. Hi again Ravi. I'm getting closer to understanding you I think, but yet again you haven't introduced yourself and your situation :-)
Are you trying to color one item for yourself or for a science project, or are you a small designer, or are doing an internship in a large corporation and looking for a high volume solution? The answer will depend on this.
A million dollar titanium nitride vapor deposition unit as suggested by Trevor and Jim, or an e-coating line as suggested by Praveen and TK are inappropriate for a hobbyist student project, but probably will be the best high volume approaches. For a smaller operation you may need brass plating as Praveen and Sara suggest, or the more exacting compositions described by Geoff. For rock bottom cost you might consider lacquering with a UV lacquer (like nailpolish) and use a UV curing tray (very cheap). Good luck, and please keep us moving forward with a detailed reply. Thanks, and good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Sir, my dad has a small jewellery shop and is doing some gold plating in small budget but we are using gold for this, so income is less. Somebody told me that instead of gold, use nordic gold. I tried but color is not similar to gold. And I know the gold alkaline making process can use same. Is there any procedure? Please help me.
ravi kumara m r [returning]student - Bangalore Karnataka India
May 29, 2014
A. Hello again Ravi. Please see
www.pfonline.com/articles/decorative-gold-substitute
where the good doctor, Arthur Kushner suggests:
- Sodium cyanide 8-9 oz/gal
- Copper cyanide 4 oz/gal
- Sodium stannate 1.5 oz/gal
- Sodium hydroxide 1.5 oz/gal
- Rochelle salts
⇦ on
eBay
or
Amazon [affil link]
6.0-6.5 oz/gal
He notes that the bath should contain between 9-12% tin and should be operated at 150-160 °F. Copper anodes are used, and varying the amount of copper and tin in the plating bath controls the color.
Best of luck,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Thanks for your reply sir, but I am in a little confusion how to use all these chemicals. I need the formula or steps. Please help me sir.
ravi kumara m r [returning]student - Bangalore Karnataka India
May 31, 2014
A. Hi Ravi. I've given you the formula you asked for, and the reference where it is found. But sorry, it is dangerous and perhaps not possible to explain in this forum all that must be explained to teach someone how to formulate and use home-brewed electroplating chemicals involving cyanide! Please see our page of recommended plating books and thumb through a couple of them and then get back to us with specific questions. Sorry, but no one but a trained chemist should be attempting to mix deadly cyanide based chemicals. Best of luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I have made Nordic Gold using copper, aluminium, zinc, tin. Good gold color came but in a few days it's getting black color. What to do? Please help.
ravi kumara m r [returning]student - Bangalore Karnataka India
June 25, 2014
A. Hi Ravi. The reference suggests this plating as an underlayment for a final gold topcoat. It probably tarnishes quickly. If you can't put even a little gold flash over it, a clearcoat might help.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Sir, please explain this line that you have written in the end of above formulation:
"He notes that the bath should contain between 9-12% tin"
a small shop - Faisalabad, Punjab, Pakistan
April 7, 2015
A. Hi Ateeq.
Presumably, Dr. Kushner means that the color will come out best if the copper and tin chemicals are adjusted so that 88-91% of the metal in solution is copper and 9-12% of the metal in solution is tin. But we gave you the link to the article; please read that short article yourself rather than relying on my interpretation of it. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
|
|
A. Try using pyrite ores. I read it was used extensively in war time to achieve gold color on glass.I heated some pyrite up in a stainless steel tube hoping to extract gold! - Trout Brook, New Brunswick, Canada Just a quick point, pyrite ores are not valuable depending on how much gold in them as they contain no gold, - Isle of Man, Great Britain |
Zinc plating and 'gold' chromating
Q. Hi I am looking for information on plating just mild steel but I want it'll be a good color; it's for corrosion protection of some parts I've made but I wish to know what is used to produce the gold colored plating found on many products to protect them from rust.
Anthony Muscat- Melbourne Vic Australia
September 2, 2015
A. Hi Anthony. There are more than a dozen commonly plated metals, hundreds of alloys, and thousands of combinations of plating. But zinc is the most common plating for steel and it often receives a post treatment of yellow ("gold") colored chromate -- so I suspect that's what you are thinking of. No one would confuse it for gold, but on cheap stuff like the innards of door latch hardware people sometimes think it's brass.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. That sounds like what I'm after. Is that stuff dangerous to work with?
And how would it be added after zinc plating?
- Melboure Vic Australia
September 2, 2015
(affil links)

free pdf is currently available from academia.edu
A. Hi again Anthony. Chromating is a simple dipping process directly after rinsing the zinc plating. When using the safer trivalent chromates (free of hexavalent chromium), it is usually necessary to follow the chromating step with a dip in a proprietary sealer or post-dip for good corrosion resistance. Production shops will sometimes do a dip in dilute nitric acid between the zinc plating and the chromating, but it's probably not necessary for a hobbyist.
If you were asking whether it's dangerous for experienced platers who've had hands-on OJT training & annual haz-mat training, and are wearing proper protective equipment, no it's not. But if you mean are there any possible dangers for an inexperienced hobbyist, his children, and his pets in trying to do an industrial process on his kitchen table, yes, certainly.
Although the most straightforward way to approach the project is to send the parts to a plating shop, we also have dozens of threads on this site about what hobbyists can do, how they can substitute more laborious operations for more risky chemical ones (e.g., scrubbing with pumice in lieu of caustic cleaning), and what they still should never attempt (e.g., cyanide-based electroplating, hydrofluoric acid pre-treatments). A very determined hobbyist who is careful can surely successfully zinc plate steel parts and buy gold-colored trivalent chromate conversion chemistry and apply it without hurting himself. You can prove to yourself that you are a determined hobbyist by going through the Metal Finishing Guidebook cover to cover -- not necessarily reading each word, but at least glancing at every page to reduce the chances of broad areas of gross misunderstanding :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi there, I am looking for a plating process which gives me a gold plated finish on my iron articles. Is there any kind of plating/passivation, etc., which is similar to gold plating finish? Kindly help.
umang goyalbusiness owner - jodhpur,rajasthan, India
March 26, 2018
A. Hi Umang. We appended your inquiry to one of many threads about finishes that "look like gold". Titanium nitride looks very much like gold but is vacuum-deposited not electroplated, and probably not suited to low volume; brass plating can look a fair bit like gold to some people but tarnishes; zinc plating with iridescent gold colored chromate looks vaguely gold-ish but wouldn't fool one person in a hundred.
If you want real help on this, it isn't going to be sufficient to be vague; rather, you must write several paragraphs explaining exactly what the parts are, what environment they will experience, why you want them to look like gold, and how many you need so we know whether very high capital costs like for Titanium Nitride deposition are feasible, what your skills are, etc. Thanks, and good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Anodized stainless steel
Q. I have a stainless steel tube, 1.715 long, .224 od, .010 wall.
Will do 15k to 25k units a month. I want to have an 14 karat gold finish look (a bright yellow color). Is there an economical process for this. Also, can my tube be plated with another coating (copper, tin, zinc, etc.) so a treatment of yellow ("gold") colored chromate can be applied? Thank You,
ink pens - MOUNT MORRIS, Illinois, USA
April 6, 2018
A. Hi Claude,
As long as the SS alloy is not 316, gold colors can be achieved by anodizing. It cannot be done in basket, but still the process is pretty economical. Please see the attached photo.


Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. How do I do brass gold on ABS material?
Is there a direct one use chemical? I want it in reddish tone.
Alpenglow - Mumbai India Maharashtra
May 15, 2018
A. Hi Narendra
Surely there are gold-tone paints that can be applied in one step. Electroplating on ABS requires at least a dozen steps.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Mirror gold/brass finish on steel/stainless steel
Q. Hi,
I am building a sculpture and ultimately the finish needs to look like mirrored gold.
It s a large piece 5'x10'x9' that will be made mostly of sheet metal, angles and extrusions of stainless steel, steel and brass.
I am currently thinking of plating the pieces of steel/stainless steel with a thicker brass coating, complete the sculpture then polish the entire thing to a mirror finish and lacquer.
Or completing the sculpture in the different base materials, taking it apart then plating them to a mirror finish if that is possible, followed by lacquer, then putting the sculpture back together.
It doesn't matter if there is a slight color variation between the plated steels and original brass pieces.
The sculpture is going to be susceptible to damage/scratching so need the option to be able to re-polish if necessary without having to dismantle it. So vacuum coatings would not be ideal I am thinking.
A friend has a metal work shop and is experienced in metals and finishing. Ideally we would like to do the finishing work ourselves. There will be many different parts and it will take over a year to build so do not mind investing in a bath set-up to install at his shop.
Is brass plating the best option considering the base materials, mirrored finish, similarity to gold, ability to repair/re-polish; and the type of work that can be undertaken ourselves without too much of an investment in equipment?
Is it possible to achieve a mirror finish straight out of the bath without polishing?
Any other considerations/options?
Thanks,
Byron
- Brooklyn, New York, USA
September 17, 2018
A. Hi Byron. A thin layer of brass plating deposited onto a shiny surface like polished stainless steel or bright nickel plating can be bright without polishing. A very heavy layer, or any layer deposited onto a non-shiny surface will not be shiny.
If applicable, brazing will be a better joining method than welding -- it's much easier to grind smooth and bright nickel plate.
It is unlikely that you will be able to maintain brightness without brass lacquer or a clear coat, however, because brass tarnishes readily.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
What gold substitute for motorbike parts?
Q. I'm contemplating plating some motorbike parts with a gold substitute. Parts include the outer heat shields of the exhaust system, air filter covers, headlight, and fuel tank dash surround.
What is the best way to do this please? Can anyone tell me what I need to ask electroplating companies near me in Perth West Australia please?
- Success, West Australia, Australia
October 29, 2018
Q. Hi,
my question is related to this thread that how can we identify that any component having either real gold plating or yellow bronze plating? As we have seen the colour of both finishes are same.
Even though the thickness of real gold is very less as flash Plating to achieve the colour and could not be analyzed by XRF Machine? If there is any consolidated method please suggest.
S&S GARMENT ACCESSORIES INDIA PVT LTD - NEW Delhi, India
March 13, 2020
A. Hello Awadhesh!
You can put a drop of 5% hydrochloric acid on the part you want to assess. If the drop dissolves the coating, it is zinc. If not, it may be gold.
XRF must give you accurate data about the coating. It should give you thickness and composition of either zinc, gold or any other metallic coating.
Hope it helps! Best of lucks!
Daniel
N. Ferraris S.A. - Cañuelas, Buenos Aires, Argentina
Q. Hi,
I have mild steel 0.8 diameter balls and want then to have a golden color look it is an imitation product. Can you suggest me the process to get it done.
I have some knowledge that it can be done in a barrel.
Please suggest.
- Mumbai Maharashtra, India
October 24, 2020
A. Hi cousin Viraj. There are many ways to get a generally golden color without using gold: from vacuum metalizing with aluminum followed by a gold tinted lacquer, to PVD coating with titanium nitride, to brass plating in a barrel, to chrome-look paint plus tinted lacquer, to CED coating and post-dyeing, to specialty gold-tone paint. Further, when you say it's an "imitation product", that by itself doesn't really rule out gold plating, as costume jewelry and imitation jewelry is usually gold plating on a cheaper substrate.
So it's hard to suggest anything until you give us a requirement & parameter or two.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi Ted,
Than you for the reply.
I am having mild steel balls of 0.8 dia. and want it to get a golden look on it.

(ed. note: photo from Alibaba of a supplier's real gold plated ball bearings)
- Mumbai, Maharashtra, India
October 10, 2022
A. Hi. Those ball bearings can be brass plated in a tone which looks somewhat like gold, but they must then be lacquered because brass tarnishes pretty quickly. What is the intended use of these brass-plated-&-lacquered ball bearings -- jewelry of some sort? Hopefully, there will be no real gold on the jewelry because a perfect match of brass plating to gold is a tall order.
You can inquire of local plating shops if they can offer you barrel brass plating, and get some samples to see if the color is close enough for your purposes. But as already mentioned, there are many different possible approaches to getting a gold-look finish, depending on your application, situation, and desires -- which we still know little about two years later :-)
And remember that real gold is NOT expensive if you can get away with a flash of gold a few millionths of an inch thick followed by a clearcoat.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi Ted,
These are balls used in imitation don't need to be original gold. Also its life doesn't need to be very long because is used by women's of a day or 2 (it is called bindi).
It needs to be able to stored for maybe 3-4 months before it starts turning black.
Below are images of product:


Sorry for getting back after 2 years. Actually truth is I had forgotten that had posted a question here.
Viraj Bhalodia- Mumbai, Maharashtra, India
October 15, 2022
A. Hi again Viraj. The least expensive way to get a short-lived golden color on this is probably vacuum metallizing. A very thin layer of aluminum is deposited in a vacuum chamber, then a tinted, protective topcoat is applied. In very high volume the coating is very cheap -- it's even used on shiny mylar balloons.
The downside is that the equipment is very expensive and unsuitable to a small shop. With some skill, and at higher unit cost but much lower capital cost, "chrome-look paint" with a tinted topcoat can be applied with similar results. Please search the site for 'chrome-look paint' or 'spray chrome'.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi Ted,
These are 0.8 diameter Mild Steel balls.
So I guess its difficult to have it to suspend them for metallizing process (Correct me please if I am wrong).
Below is the process that you are suggesting right?
www.pftechnologies.com/solutions/vacuum-metallization/
My output requirement is around 5 tonnes a month (may increase later).
Oblique Barrel plating is one way to get this volume production is what I have learnt from few Electro-plating machine manufacturers. But the process and chemicals required to get it done is what I am unable to find.
One of the vendor has suggested that balls need to be turned in to silver color first and then golden look can be achieved.
Can you please share some knowledge on this.
Thank you for the time and suggestions.
Really appreciate it.
- Mumbai, Maharashtra
October 17, 2022
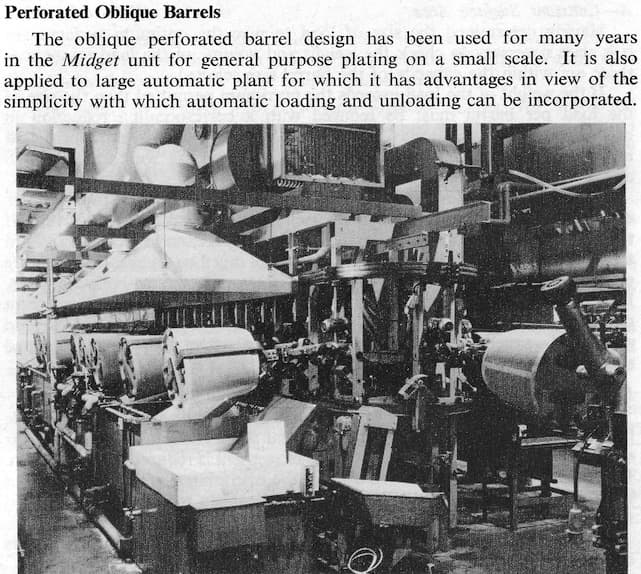
A. Hi again. If you prefer to do electroplating rather than vacuum metalizing, that's fine. This can be done in a series of individual oblique barrel plating stations, or in an automatic oblique barrel plating machine as illustrated (courtesy of the Canning Handbook).
The Electroplating Engineering Handbook ⇦ this on
eBay,
AbeBooks, or
Amazon [affil link]
as well as the Canning Handbook ⇨
have good chapters on the topic, but your proposed production volume doesn't approach justifying an automatic oblique barrel plating machine.
Today it's more common to use horizontal plating barrels anyway, either transferred from tank to tank with a manual hoist on an overhead rail, or automated with a programmed hoist (or even manually if very small barrels are used). See our library article on barrel plating. I imagine that you will want plating barrels much smaller than those illustrated (because they could easily produce your month's production in a single 8-hour shift).
I believe that the talk you've heard about "silver color" may have to do with the fact that brass plating (as well as gold plating) don't have much shine if applied directly to steel. The shine is usually produced by an underlayer of bright nickel or white bronze plating.
I suspect that you want a small and simple manual barrel plating line including the steps electroclean, acid activate, white bronze plate, and brass plate, with rinsing between each. If I were you I'd consider finding a finishing consultant. I no longer do consulting work, but something I learned from doing it for 25 years or so is that the early decisions are the most important ones, but clients usually don't retain a finishing consultant until near the end ... after the freedom to optimize the plan has become severely limited.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I am trying to make a steel sword appear gold.
I tried electroplating over nickel and I'm guessing you're all having a laugh now. Lol. It was not successful.
It won't get used in battle. It's a friend of mines who just turned 25 and purchased this sword for that golden anniversary. And it will be a wall display and at most used for Ren-fair costume.
The handle and other bits actually started to dissolve in the electroplating solution of vinegar and gold I was using. I think it was the combination of air pockets and strange alloy. Any advice on that as question 1?
And, how can I make this steel look like gold. I've given up trying to actually use gold that I now have to try to reclaim from my ionized vinegar.
I like the idea of applying aluminum over the surface and anodizing that but it's there something better you would recommend instead?
Hobbyist - Elmira, Oregon US
November 6, 2022
A. Hi Jason. Unfortunately, applying aluminum over the steel is not something a hobbyist is likely to be able to do nor have a plating shop do at an affordable price.
I think the best approach for a decorative sword will be "chrome-look paint" with a translucent gold-tone top coat. These "paints" are not the hardware store gold-tone paints you might envision. Rather, they are a 3-coat (or more) system where the base is a tailored primer coat, the middle layer is done with silver nitrate plus a reducing agent to leave a shiny chrome-like look (essentially the 'mirror silvering' process), and then a topcoat which is often clear but can be tinted. You can contact Gold Touch [a finishing.com supporting advertiser] and G.J. Nikolas [a finishing.com supporting advertiser] about it, or google for "chrome-look paint" or "spray chrome".
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Gold leaf
A. Try gold leaf or imitation gold leaf (cheaper). Or try ferrocyanide based gold plating bath (if your sword is ordinary steel you can gold plate it with ferrocyanide based bath directly). Potassium ferrocyanide ⇦ on eBay or Amazon [affil link] is non-toxic cyanide compound. Hope it helps and good luck!
Goran Budija- Cerovski vrh Croatia
![]() Hi again. I thank Goran because the possibility of gold-leafing slipped my mind and I think that idea is better than mine was :-)
Hi again. I thank Goran because the possibility of gold-leafing slipped my mind and I think that idea is better than mine was :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread



