
Jump to related thread
• Topic #49751 "White Bronze Plating" or
• Topic #1114 "Bronze Plating Solution Make-Up, Parameters, Analysis, CN v. Pyrophosphate"
• or continue here with ... -----
Bronze Plating Q&A, Problems & Solutions
Quickstart:
Bronze in an alloy of tin & copper. As with all alloy plating, one of the two elements is more 'noble' than the other so, due to electromotive potentials, the natural tendency of the plating solution would be to deposit the more noble metal nearly exclusively even if the concentration of the less noble one greatly exceeds it.
To deal with that problem, alloy plating processes incorporate powerful complexing agents (often cyanide), and are usually proprietaries not appropriate for hobbyists.
Keeping the solution in balance for a long time can be difficult.
Certain substrates may act as catalysts triggering imbalances in an alloy deposit, so alloy platings sometimes require first plating the substrate with copper, nickel, etc., before the alloy plating.
Heavy Bronze Plating Is Delaminating
Q. I make an hydraulic piston shoe that has a heavy bronze electroplating on it with a nickel strike between the 4140 steel and the bronze. The steel is hardened to 44Rc, and before plating has a surface finish of 12Ra-15Ra.




The bronze has delaminated at the nickel strike. There is nickel on the steel and the bronze, what would cause this?
John CushmanEngineer - Lebanon [Ohio]
May 21, 2025
A. Hi John.
If there is nickel on both surfaces, the problem appears to be in the nickel strike process. Nickel is well known for laminar plating if the contact is interrupted and the nickel plated surface goes passive and then more nickel is plated onto it.
Is that a possibility? Was this a one-time incident or is it a continuing problem? Is the nickel strike a Wood's Nickel? Thanks.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I thought i would add a few more pictures of the Nickel strike layer. these are SEM pictures at pretty high magnification.


On the parts that have delaminated, they all show voids or porosity of the nickel strike.
John Cushman [returning]Engineer - Lebanon [Ohio]
A. 4130 has a bit of chromium in it and you might want to look at your pretreatment cycle. You probably should have some type of electrolytic acid to help bite into the steel for better adhesion. The pores in the nickel plate could be from poor activation of the substrate.

George Shahin
Atotech - Rock Hill, South Carolina
June 23, 2025
Q. George,
The problem is that we are not doing any of the plating. Our subcontractor it pretty tight lipped about the process and the pretreatment. I'm just trying to get some possible failure modes to suggest. They should be able to re-create the issue, but so far they have not been willing to do any experimentation to determine a root cause.
Engineer - Lebanon [Ohio]
June 24, 2025
⇩ Related postings, oldest first ⇩
Q. I am facing problem in process of plating on brass. I want to finish yellow bronze over it. Can any body suggest me the actual process. Please help.
I am a chemical Engineer running my own industry at Delhi, India. This time I am doing job work for watch and Sanitary industry giving gold, silk and matte finish. I have develop various color. For one of my project I need bronze/yellow finish over brass. I have done similar color but it is not accurate that much I want. Can you help me in this matter.
- DELHI, India
2002
Ed. note: Robert Probert explains some coloration issues and approaches below.
A. I am having some difficulty in understanding the question. Is it the antique finish you are looking for? For colors over brass you can also try electrophoretic lacquer with dye.
Gurvin SinghMohali, Punjab, India
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Would anyone have information on bronze electroplating systems?
Where they can be bought, how do they work, etc.
Thanks, Joe.
metal fabrication - Tallahassee, Florida
2003
A. Hi Joe. Bronze is one of a few dozen metals and alloys that can be electrodeposited. It can be plated for both functional and decorative reasons. There would be a number of preliminary cleaning and acid activation stages on the substrate, then the plating step proper, then possibly some tarnish preventers, or lacquers, etc.
You can buy amateur level plating systems for a few hundred dollars, or a professional level small brush plating system for a few thousand dollars. But you can also spend a million dollars or more for a fair size automated bronze plating installation. Please tell us a little about your needs and situation, so we can continue the discussion most productively.
Good luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. "Bronze" means different things to different people.
To the metallurgist bronze is an alloy of copper and tin.
To the architect or plating job shop, bronze can be a "color". To get the particular shade of color you need, you may experiment by mixing copper plating solutions with zinc plating solutions sometimes having to add a bit of an alkaline tin. You might also get the color bronze you need by merely increasing the copper content in a standard brass plating solution.
In the 1950's some architect in Atlanta specified a particularly difficult shade for the wall light switch covers in the restoration of the state capitol. As a co-op student from Georgia Tech working at a dirty job shop, I was able to get his color by blending my standard brass solution with our standard alkaline stannate tin solution.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. I would appreciate being led to bronze electroplating procedure.
Majid Ghahari- Tehran, Iran
2003
A. Hi, cousin Majid. We have a number of threads on line here that specifically address bronze electroplating, including this one and:
Are you looking for a heavy functional bronze for engineering purposes or a decorative layer on top of nickel plating ... a handful of parts or a major installation? It's difficult to distill it down to a few paragraphs until you tell us your situation. Are you working for an established plating shop and experienced in many different plating procedures like brass, but not bronze . . . or have you not electroplated at all yet and need an intro to electroplating in general first? Thanks!
Best of luck and Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Bronze Plating Problem: Tin-rich layers are forming
Q. We are trying to plate out bronze (8-15% Sn) 0.022 thick on small disk shaped parts for wear. We use a potassium/cyanide electrolyte solution and copper anodes. We have a problem with layering of tin-rich zones that typically begins after the parts have been plating for some hours, that becomes so pronounced as to ruin the parts. However, this problem comes and goes, and often the parts are just fine. We use constant current density. Does anyone have an idea as to what causes this layering?
William DirkinManufacturer - Kalamazoo, Michigan
2004
A. Hi William.
It's important to remember that being able to electrodeposit an alloy at all is quite a feat :-)
Copper, being more noble than tin, would be expected to plate out exclusively in a bath where ions of both copper and tin are in solution. The way it becomes possible is by restricting free ions to near zero by complexing/sequestering the metals with the right amount of caustic, the right amount of cyanide, the right amount of tin salts, etc.
Lots of things can go out of balance and affect the copper-tin ratio when you are plating for some hours, but Lowenheim notes in Modern Electroplating ⇨
that higher temperatures increase the ratio of tin deposited. Assuming you don't have cooling coils in the tank, is it possible that the temperature is not being monitored but is rising over the course of those "some hours" due to the plating current being input?
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Bronze plating on steel plate for exterior use?
Q. Looking for advice on plating techniques for outdoor metal finishes. I'm an architect and furniture designer, and I'm working on a bench design that uses metal structurally. I want it to be dark in color, like bronze or gun-blued steel. But I also want it to be thin, so structurally I need to use steel. (We looked into bronze, but it would need to be 3/4" or 1" thick plate - heavy and expensive!) Is it possible to have mild steel plated in bronze? Will that be a durable finish when exposed to weather? (Obviously we don't want the steel to rust.) Or any other ideas for dark patinated finishes (not paints or powdercoats) for exterior metalwork? Thanks!
Peter Larsenarchitect - San Francisco, California, USA
2005
A. Copper plating with oxidised or patina finish may be the only viable option for architectural use.

Khozem Vahaanwala
Saify Ind
Bengaluru, Karnataka, India

A. We thank Khozem for his very knowledgable reply, but also add in case the implication wasn't understood ... you are going to need a clear coat on it.
The only finish I can think of that would be this color and suitable for outdoor exposure without a clear coat would be nickel and chrome plating followed by a colored PVD nitride coating such as the "lifetime finishes" used on door hardware, but the cost sounds highly impractical.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Bronze / Brass that looks like Gold?
Q. I want to give gold effect on artificial jewellery.Fine gold is very costly. So, I want to use Yellow Bronze Plating. If someone can help me, giving workable formulation. I don't want to use any proprietary chemicals.
Thanks,
Electroplating shop - Lahore, Pakistan
2006
A. Zafar,
yellow bronze does not give gold effects. Try gold dyed lacquering.
Regards

T.K. Mohan
plating process supplier - Mumbai, India
A. Hi Zafar. In topic 37526, Geoff Smith describes Pinchbeck Alloy, Dutch Metal, and Nordic Gold -- brasses designed to look a lot like gold. That should be at least a start.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi My Name is KISHORE. Actually I got a problem with my plating unit -- it is bronze plating. When I am starting plating arranging desired current, but if it would be down 0 current and also plating surface getting straight lines after plating, so kindly solve my problem. Mostly I like to know the testing procedure to get the Bronze salt-a, salt-b, Potassium cyanide and Potassium hydroxide in the chemical.
thank you..
shop floor manager - Hyderabad.Ap&India
August 29, 2009
A. Hi Kishore. We may have language problems, or keyboard conversion issues; I can't understand your question-- apologies. But it is usually much better to buy a proprietary bonze plating process from a plating process supplier so you can get local, hands-on help.
Readers will probably be happy to help, but we would need to quite a bit of data like concentrations, amperages, temperature, etc., from you to try to guess what is wrong. And if you could e-mail a pic of "getting straight lines after plating", that would probably help a lot!
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Bronze plating problems on O6 Tool Steel
Q. My company manufactures and repairs aircraft hydraulic parts. Over the last several months, we have been noticing that our bronze plated parts are being attacked by some sort of chemical reaction that can best be described as black "smudging" and pitting on the bronze plated face. The problem presents itself after the parts have been installed and run on a test stand. The operational environment of the parts is characterized by high rotational speeds and high oil temperatures (225 °F-240 °F). We have manufactured these parts for 10 years or so and have just now experienced this issue. We have tried three different plating vendors and have had the same results. This leads me to believe that there may be an issue with the base material (O6 tool Steel). Could the graphite content of the tool steel be drawn to the surface of the part during the etching phase of pretreatment and become included in the bronze plating itself? If so, would this cause an adverse reaction similar to what we have seen? Thanks.
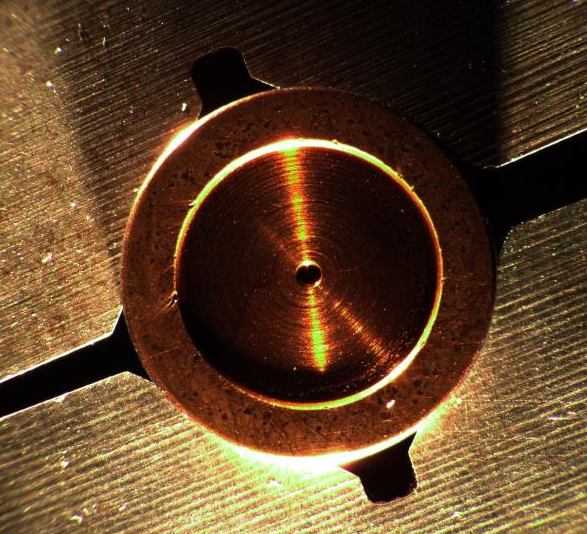
- Agoura Hills, California
December 14, 2011
A. Hi Warren.
I don't have an answer as to whether graphite inclusions are acting as some sort of catalyst throwing te deposit out of balance and causing lighter/darker areas, but have a suggestion for a trial. Some alkaline solutions, like zinc, have difficulty plating on high carbon steel. I wouldn't expect it with tin or copper, but alloy plating can be tricky. Since three shops have failed to plate the material correctly I'd suggest having one of them try a nickel strike before the bronze plating if there is no reason you can't.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I'd like to know differences between bronze plating and brass plating.
Currently I'm using brass plating to do antique brass finish, but mostly I'm not able to match the antique finish as per the sample I receive from clients. How can I adjust the colour in brass plating, like more yellow or antique brass. Many times I fail to match antique finish colour. Please advise.
- Dubai, U.A.E.
January 28, 2012
A. Hi, Shahid.
We appended your inquiry to a thread where Robert Probert suggested that if you're good at it and patient it may even be possible to simply mix an alkaline brass and an alkaline tin bath for some different colors. The previously mentioned topic 1114 notes that you can buy the solutions from Macdermid or other vendors, and also includes some formulation information.
Despite these options though, you might consider trying to get the desired color via electrophoretic lacquering, or blackening & clearcoating on your existing plating if color is your chief concern.
Regards and good luck,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Can a yellow bronze plated object be successfully plated with gold plating?
Mr. Bhupendra Upadhyay- Mumbai, India
April 2, 2012
A. Hi Bhupendra.
I don't see why this should present a problem, but an intermediate layer of nickel is probably desirable if you are looking for a bright gold plate; otherwise, an intermediate layer of copper and probably a gold strike might be necessary. But answers to questions with little context can be misleading because there are so many possible "ifs, ands & buts" that can't all be covered in every forum answer. So please tell us as much about your actual situation as you can for the best answers. Thanks.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q.
Hi folks,
I have been sent some 'gold' coloured buttons (from some sort of garment I believe), there is a customer complaint regarding tarnishing and having looked at them they do have brown patchy tarnishing in certain areas, maybe approx. 10% of surface area in seemingly random patches, looks like brass tarnishing.
I have analysed them microscopically and using XRF and they are a 60/40 brass with approx. 2 microns copper underplate and what appears to be a yellow tin bronze (quite gold coloured) which is approx. 1 micron. there is no lacquer coating.
I was wondering what the general tarnish resistance of yellow tin bronze is? I think it is pretty good but not as high as the white bronze?
I assume copper will migrate into the thin bronze plating (and tin from the bronze will migrate into the copper)? This will lower the tarnish resistance of the bronze plating I assume? Any idea how long does this take at room temperature, are we talking just months or years? And could copper migration be a cause of the tarnishing, would it very significantly lower the tarnish resistance?
I think a typical yellow bronze is something like 80% copper, 10 -15% tin and 2.5 - 5% zinc, that correct?
I was wondering what to tell the customer as an explanation for the tarnishing, and how big an effect this copper migration may have. The bronze plate is only about 1 micron but that should be thick enough to be reasonably coherent and not porous I assume. I don't suppose there is a significant galvanic effect between bronze and copper.
The actual environment/chemicals, etc. the buttons have been exposed to and how long the buttons have been in use is relevant, but unfortunately that info wasn't provided.
Well, any help/comments would be greatly appreciated.
Steve
Technologist - Sheffield, UK
March 13, 2013
|
|
Technologist - Sheffield, UK March 17, 2013
My guess is that the problem might be cyanide bleed-out, but I do not have sufficient bronze plating experience to answer your questions, and verbal descriptions of defects are not always correctly understood. Thanks & Regards,  Ted Mooney, P.E. Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted can be retained for immediate answers or long term project help |
Bronze plating line producing what looks like immersion tin and causing peeling
Q. I am a chem engineer running a bronze plating bath. We have had a dramatic ramp up on scrap peels, but only on specific substrates (we run multiple steel alloys through this bath).
One of the things we're seeing is an immersion tin layer that is depositing before the bronze and inhibiting bonding. What may be causing this condition and how do I stop this? I'm not even sure where to begin looking.
About the same time as our scrap spike, we had a couple of changes in our process.
1) We added a water softener/RO system to our already-existing DI water filter system. The water we are using is still DI, just now - instead of direct from well, the water is "pre-treated through the softener/RO before the DI filters.
2) We moved our rectifiers on our electroclean tanks (after being stationary for 40+ years). All the leads & connections were replaced new.
Is there anything specific that I should be looking for?
Any help is appreciated!!!
Thanks,
Dawn
- Bellaire, Michigan
April 20, 2016
A. Hi Dawn,
The one thing that immediately comes to mind when you mention problems only with specific steel substrates is that leaded steel will surely give you a problem if it's not specially activated with a fluoride-bearing acid. Might any of the steel be leaded?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi. I use the "makeup solution" in the plating workshop to make the bronze bath. Does anyone know the nature of this solution? Thank you.
Fateh heydari- Iran
December 9, 2019
A. Hi Fateh. Bronze is an alloy of copper and tin, so those two metals are probably in the makeup solution, and most bronze plating baths are cyanide-based. Parthasaradhy ⇨
suggests copper cyanide, potassium stannate, potassium cyanide, potassium hydroxide and Rochelle salt
⇦ on
eBay
or
Amazon [affil link]
. Copper is complexed by cyanide, and tin by hydroxyl, making it possible to control the ratio of copper and tin that are plated.
Lowenheim's books, both "Electroplating" ⇦[this on eBay , Amazon, AbeBooks affil links] and "Modern Electroplating" [on AbeBooks, eBay, or Amazon affil links] offer good coverage of bronze plating including formulas. Do you know whether your anodes are bronze, copper, or what? Please tell us about your situation and why you what you mean by the "nature" of the replenishment solution -- your question might be simple and generic, but we can't participate in crowd-sourcing of proprietary information. Thanks!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
RFQ: Plating for watch components: I have several watch cases that I want to use to build better watches. I'm starting with a large case that is "bronzed" but I want a good bronze plating on it. The dial is apparently unique (size and shape) and I have to have a new one custom made and want it bronzed also; but then lacquered to prevent oxidization while I want the case to age.
(After this will come a large chronometer and a stupid green case that I love the texture of and I think it would look great in rose gold. These are 1 at a time projects, but there are several and I know there will be more as I break down what I have into good and garbage).
- Lehigh Acres, Florida
December 12, 2020
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
Q. I have a Cincinnati Victor Luminaire funeral fan from the 1920's that has a bronze or orangey copper color plating. I'm looking to re-plate and restore the finish because the cast iron has lost a lot of finish.
Does anyone in the USA do Bronze plating or can match a color?
Can someone share some advice how I can use my rectifier and plate it myself to get a close color. Also I was thinking copper plating and chemically changing the color but not sure what chemical can change copper to a bronze color.
To see color, look up Luminaire funeral fan under images on the web.
- Folsom, California
July 7, 2021
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. Hi James. Earlier on this page Robert Probert describes how to adjust the shade of the plating but, unfortunately, cyanide bronze plating is not for home or amateur use because cyanide is such a powerful poison that it can't be used without training, equipment, and proper facilities. A man in my town died doing cyanide plating on his kitchen table, and the first responder was hospitalized. There are many plating shops which offer bronze plating, but identifying those which are set up to handle consumer items like this rather than high production runs may take a lot of googling and phoning.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
this text gets replaced with bannerText

Q, A, or Comment on THIS thread -or- Start a NEW Thread


 Right, well that's all a lot clearer now. Thanks for the help.
Right, well that's all a lot clearer now. Thanks for the help.