
Home of the world famous 'finishing.com HOTLINE' since 1989
-----
Hard chrome plating: Volts, Amps, Anode-to-Cathode Distance
Q. I have a hollow cylinder (SS446) the diameter of the cylinder is 1 inch and the length is 3 inches.I need a hard chrome plating internally, which type of anode I will use and shape also, total time for plating (50 micron), total current and voltage, etc.

Student - Pakistan
March 19, 2024
by Robert K. Guffie

on eBay (rarely) or Amazon (pricey)
or AbeBooks (rarely)
(affil link)
A. Hi Ahmed,
First calculate the surface area of that I.D. you want to plate. Then the required amperage will be an absolute minimum of 1 amp/sq.in. of surface area (chrome, unlike some other metals, will not plate at all if the current density is too low), with 2 amps/sq/in. probably being a good value.
Hang the part vertically so the hydrogen you will be generating can most easily escape. The anode should be a lead rod centered in the I.D., with somewhere around 2-1/2" of exposed lead and the rest masked to prevent dog-boning at the ends of your 3" length.
The voltage will be whatever is required to hold the 2 amps/sq.in. but probably well under 6 volts.
You should calculate the required plating time from Faraday's Law, assuming probably about 10-12% efficiency unless you are using a HEEF-25 bath, in which case something closer to 20-25% can be assumed. Advise if you don't understand Faraday's Law or efficiency.
You will need to clean, and then either Wood's Nickel Strike or etch the stainless steel before it will be plateable.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi sir, what anode diameter I will use?
Masking area of the tube, if the length of the tube is three inches.
Sir please tell me formula to calculate total time for 50 micron to do.
I didn't understand the masking length like internally tube masking or externally and which length.
- Pakistan
March 20, 2024
(affil links)

free pdf is currently available from academia.edu
A. Hi again, Ahmed.
If you were doing production plating the diameter of the anode would matter towards controlling the gradual build-up of trivalent chromium. When you're a student or hobbyist doing one or a few items, this isn't very important; if you had a lead rod or tube of 3/8" or 1/2" diameter, that would be fine.
I gave you the formula to calculate the plating time (Faraday's Law with efficiency of about 10-12%), and asked you to advise if you didn't follow it. But there is a chart on page 866 of the on-line version of the Metal Finishing Guidebook ⇨
which does most of this calculation for you, simplifying it down to the fact that it takes 51.8 amp-hours of electricity per square foot (at 100% efficiency) to deposit 0.001" of thickness. Since the efficiency will be, say, 10%, it will take 10X that many amp-hours. You get plating time by dividing amp-hours per square foot by the amps per square foot you are plating at. I previously advised that should plate at about 2 amps per square inch (or 288 amps per square foot. So the plating time for 0.001" thickness would be 51.8 hours x 10 / 288 = 1.8 hours = 108 minutes. You can scale down to 50 microns (0.2 minutes) or any other thickness.
You said you wanted to plate "internally", so the only anode will be internal, running through the center of your item. If you were to use a bare anode, you would get a little bit of plating on the outside of the tube near the ends; so you would want to mask the anode except for the 3" portion within the item you are plating. Even then, the plating would be a bit thicker near the ends, so we usually mask a little bit more, exposing only, say, 2-1/2" of anode. But for a first time effort, masking the anode and other fine detail is probably overkill :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇩ Related postings, oldest first ⇩
Q. Please tell me more about hard chrome plating, for example the ideal current density, voltage, temperature, solution percentages, etc.
I'm starting a hard chrome plant for rollers and plastic molds.
Thanks for any suggestions.
Remberto BritoReproqui S.A. de C.V.
1998
A. Hi, Remberto. We're always delighted to answer questions here, but you should probably start your investment in your chrome plating operation with the purchase of some books on the subject, and hiring an experienced chrome plater and/or retaining a finishing consultant to get you started because a few paragraphs will prove a very poor substitute for the broad knowledge you will need to bring to this enterprise.
"Hard Chromium Plating" by Robert K Guffie is certainly the indispensable premier resource on this subject, but "Chromium Plating" [on eBay, Amazon, or AbeBooks affil links] by Weiner & Walmsley is very valuable too. For general historical background "Electrodeposition of Chromium" Electrodeposition of Chromium from Chromic Acid Solutions ⇦[this on Amazon affil links] by George Dubpernell is informative reading (although I've personally come to feel that it overstates the importance of M&T Chemicals and understate the contributions of everyone else :-)
1). There are at least three very different general types of plating baths based on their catalysts (1. sulfuric acid - the traditional 'Sergeant' bath; 2. fluoride - such as the 'Self Regulating High Speed' bath; 3. proprietary catalysts - such as the High Efficiency Etch-Free bath). Please see thread 35184, "Hard chrome plating catalysts" for an introduction to that topic.
2). A large range of chromic acid concentrations are employed from about 28 oz./gal to 54 oz.gal.
3). A number of different general approaches are used, for example, tank plating with stick anodes vs. Peger's reversible rack system.
4). Rollers are sometimes plated vertically, and sometimes plated horizontally while slowly rotating, with the bottom half (or so) submerged and the top half above solution level.
For these reasons,
- the ideal current density may range from a low of about 1-1/2 amp/in^2 to 3X that and more.
- the voltage is closely proportional to the anode-cathode spacing and solution conductivity, so it might actually be only 3-4 volts with a clean solution and close spacing or it might be 12 volts (maybe even more) in some cases.
- the temperature is usually between 130 and 140 °F, but again it can depend on the other factors.
- the concentration of chromic acid is usually, as previously mentioned 28-54 oz./gal. with 32 oz./gal. probably being most typical. But there will always be catalysts in the general range of 1% of the chromic acid concentration.
You might get hold of old issues of Metal Finishing magazine and read what Clarence Peger -- a strong proponent of reversible rack plating -- has to say. Best of luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
by Clarence H. Peger

(copies are avail. in select libraries)
Very rarely available from
eBay or Amazon
or AbeBooks
(affil link)
A. In addition to looking at past issues of Metal Finishing magazine for Clarence Peger's articles about chrome plating, get a copy of Clarence Peger's books about chrome plating.
Hard Chrome Plating Simplified (CPSR) - a manual that consists of basic, simplified, hard chrome plating information to help you make greater profits; with over 200 illustrations and photographs; also includes 32 blue prints. 382 pages.
Hard Chrome Fixtures II (BLFT) a manual consisting of 80 full-color photographs of chrome shops, racked work, and other related images, 15 black-and-white photographs, 8 line-drawings, 5 papers, back issues of Hard Chrome Plating news letters, and other informative articles. Each photo has a commentary. 300 pages.
All of the information in these two manuals is based on a proven high-speed chrome plating method that produces even deposits at 0.006 + per hour plating rates. These manuals have been called the Hard Chrome Plater's Bible and the Cook Book of Chrome. If you hard chrome plate, you must have these manuals!
The manuals are available from:
Hard Chrome Plating Consultants , Inc., Cleveland Ohio
Mary PegerCleveland, Ohio
Ed. note: We're delighted to see you carrying your father's legacy forward, Mary!
In general we can't post advertisements in these forum responses (huh? why?), but out of great respect for your father's contributions to the subject we've posted your reply.
Please consider becoming a supporting advertiser so you can advertise on this site; otherwise it gets very hard for us to justify printing your promotions while dismissing as spam the promotions of others.
"The Demand Curve for internet advertizing:
Infinite demand at zero cost, zero demand at infinitesimal cost ![]() "
"
-- Thomas J. Pullizzi, co-founder of finishing.com, 1995
Q. Hi guys, Can anybody give me some hints of the operation costs of running a chrome plant?
Rolando Riley- Panama
2008
A. Hi, Rolando.
As with many businesses, labor cost is surely the biggest single item. It takes several hours (occasionally several days) in the plating tank, and hard chrome plating jobs often require careful masking, building of specialized auxiliary anodes and thieves, etc.
Electricity costs can be substantial: look into Faraday's Law to estimate electrical usage based on the amount of chrome you will deposit, remembering that the chrome is in hexavalent form, and deposition efficiency is probably only about 12 percent. The air exhaust system also uses substantial energy.
The chromic acid itself is a fairly minor cost, but it can be expensive to manage the handling of chromic acid (hexavalent chromium) to avoid worker exposure and pollution of the environment.
Although it might not be easy, or even possible, it would be best if you very clearly understand chrome plating from visits to several chrome plating shops. Before you try to estimate the operational cost, it's useful to know what operations are actually involved and in what proportion. There are many plating shops which make good money ... but there are no plating shops which produce substantial rejects and make money. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. We plan to install 10000 amps RECTIFIER for HARD CHROME PLATING
I am confused whether to select 0-8V or 0-12V output voltage
Please advicse
PLATING SHOP EMPLOYEE - KOLHAPUR, MAHARASTRA, INDIA
June 7, 2009
A. Hi, Mikki. If you have experience in this and you never use more than 8 volts, you should get an 8-volt rectifier. Simply, there is no value whatsoever in oversizing a rectifier :-)
But if this is a new installation and you are asking how much voltage an unspecified part will require, no one can answer exactly. But essentially the required voltage is proportional to the anode-cathode distance. If you use only very closely spaced conforming anodes, 8 volts is probably enough; if you do 'tank plating' with the anodes several inches to the better part of a foot from the part you are plating, it is probable that you will need 12 volts (maybe even more). The previously referenced book by Guffie suggests thinking very carefully before considering 9-volt rectifier because real-world contaminated plating solutions will require more voltage than new solutions. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Multiple threads merged: please forgive chronology errors and repetition 🙂
Anode cathode distance in hard chrome plating
Q. I find that the closer the distance between the anode & cathode (the work piece), the lesser is the required current (Amps/dm 2) to plate a particular thickness for a given surface area.
Could you help me to understand the theory behind this.
Electro Plater - Bangalore, Karnataka, India.
December 16, 2010
|
|
A. Other than you have to add 6 electrons for every chrome atom deposited, you have a very inefficient bath. Since the voltage is above both the hydrogen and oxygen overvoltage potential, you will use a good bit of electricity to have that happen. Next, the solution will generate a lot of heat, which is more lost electricity. When you move the part closer to the anode, you will generate less heat. If you turn down the voltage to lower the amperage, you will generate less hydrogen and oxygen. Result--You use less amps to plate the same thickness. - Navarre, Florida A. RESISTANCE OF HARD CHROME PLATING SOLUTION IS VERY HIGH. Muhammad Umair Kahan- Lahore Pakistan |
Q. I have a Cylindrical part OD 38.50 mm & Length 205 mm. I have problem in chrome plating. I have No Idea For JIG & FIXTURE Design for this part.
When I try to do plating to this part/job down side is thickness variation. Suggest me how to do plating on this Part.
- Gujrat, India
April 18, 2013
A. Hi Gopal. Are you racking this part vertically or horizontally? If vertical, it's possible to arrange anodes in a ring around the part and get reasonable consistency of thickness. Anodes shorter than the part may help avoid dog-bone effect.
When you say you have "no idea" I'm not sure how literally to take that. If you are an experienced hard chrome plater looking for a tip based on this one particular shape of this part, hopefully someone can help you. If you literally mean that you have no knowledge at all of jigging & fixturing for hard chrome plating, you'll need to read some books or attend some classes as that would be way beyond what anyone can tell you via a public forum posting. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Dear sir
I set job in Vertical & I have already 1 plant.; this is my second plant. I purchased this plant for this job: it's automotive part used in heavy truck; it's called KING PIN.
I need thickness 75 micron per side total 150 micron ovality & taper maximum 30 to 40 micron.
When I try this job for chrome plating and after checking this part, Ovality & taper 80 to 190 microns.
Thanks & Regards
- Gujrat, India
A. Hi Gopal: It is kind of difficult to help you without seeing a diagram or something about the part, but I am going to give you a few hints based on my experience. Hard chrome plating is a very tricky business, it is very complex and the bath is very poor in throwing, for every 100 amps you are "really" using 12 to 14 amps, with your bath in top shape. You should use 2 to 4 amps per square inch of surface to plate well.
Put your part vertical in the tank ; jig it up with heavy copper bar or hook; hopefully you have threaded holes on both ends of your part; use a stack of steel washers or nuts (to thieve the high current density of both ends, so you can get better distribution of the layer of chrome); Like Mr. Mooney said, set your anodes in a circular way around the part (use a heavy copper ring for your cell) and keep your part in the center about 3" from the anodes. Make sure your cleaning and activation process is good, good rinsing and give it a shot. This the best I can do for you. Good luck.
- New York, New York
Chrome plating draws too little amperage
Q. Hello, I have a question concerning hard chrome plating. Over the past summer I have experienced a gradual loss of amperage on large parts. Example, if my part called for 10500 amps at 15 volts , I have slowly lost amps and am now around 7000 amps at 15 volts. My chemistry checks out good and temperature is good too.
Now here is what puzzles me. Through the summer I had slightly strengthened my chrome from 23-24 oz per gal to 26-27 oz per gal, disassembled mostly all copper contacts to clean mating surfaces and put in new anodes.
Now I thought this would give me an increase in amps but the decrease occurred. Does this mean that it now takes less amps to achieve the same end result? Or should I be looking into a problem in the rectifier?
Any info would be greatly appreciated.
chrome plating dept. - Chesterton, Indiana, USA
November 1, 2013
A. It seems unlikely that anything you did reduced the required amperage because that is dictated by Faraday's Law. With half the current, you'd put on only half the chrome, and I think you'd have noticed that thinness ... so I suspect the rectifier readings. The first and easiest guess about the problem is the ammeter and shunt leads.
The ammeter on your rectifier doesn't really measure amperage :-)
What actually happens is that there is a precisely sized block of copper alloy in your bussing called the shunt. When current flows through the bussing there is a voltage drop across the shunt just like any of the other bussing -- except that the shunt was sized to have a fixed voltage drop, usually 50 mV, when the rectifier's maximum current is flowing. At half current the voltage drop will be 25 mV, etc. So your ammeter is actually a 50 mV voltmeter. If that voltmeter is defective or the shunt is the wrong size for the rectifier rating or the meter, or even if the shunt wiring is loose or the connections dirty, the ammeter can read wrong.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Hi Pete,
I agree with Ted on his suggestion.
However you did not comment on the condition of your part.
Is the result same in same time or do you observe a difference? To my guess check your rectifier for a broken carbon leading to less current. Moreover in case you have a broken carbon it means that you are working on 2 phases which could harm the rectifier and the deposit too.
ALL THE BEST.

Vikram Dogra
Irusha India - Chandigarh, India
Q. Hi
We have water-cooled IGBT based rectifiers for our automatic hard chrome plating line -- 12 V and 18000 A.
ACRONYMS:
IGBT = Insulated Gate Bipolar Transistor
Over the past six months, whenever the demand of parts current exceeds 70 - 75% of the rectifier current capacity, fluctuation of current happens and also the actual current observed at that 70 - 75% limit is 500 - 2000 A less than the set current.
The rectifier manufacturer says that constant current output should be obtained @ 85 - 90% of the rectifier capacity but is unable to diagnose the problem.
Our chrome plating bath uses HEEF-based chemistry, with chrome concentration of 250 gpl and trivalent chrome - 7 gpl, Fe - 9 gpl. Operating temperature is 60 °C and current density is 50 ASD. We use 2.5 meter length lead-tin anodes. We have checked the busbar connections from rectifier to tank and is ok.
If rectifier is the problem, what are all the variables to be checked, to confirm problem with the rectifier.
Thanks for your assistance.
- Bengaluru, Tamilnadu, India
July 14, 2017
by Robert K. Guffie

on eBay (rarely) or Amazon (pricey)
or AbeBooks (rarely)
(affil link)
A. Hi Ezhil. If this problem developed as the plating solution aged, please consult Guffie for detailed comments on why this may happen with plating solutions as they accumulate impurities. An oscilloscope will show the actual voltage being delivered, and when sync'ed to your 50 or 60 Hz power, it can be very helpful towards revealing whether the rectifier is malfunctioning (these days some high-end digital multimeters can simulate an oscilloscope, plotting the waveform of the output voltage on an LCD display).
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. How to calculate amperage and voltage for hard chrome plating?
I want to run a small shop of hard chrome plating in guns barrels and other part of guns.


Anyone there who can help me I will be very appreciative.
aziz ullah- peshawar Pakistan
March 24, 2021
A. Hi Aziz. Chrome will not deposit at all at low current densities and will burn at too high a current density. You will probably want to size things for around 250 to 500 Amps per square foot (25-50 Amps per square decimeter), although these are not firm limits.
Voltage is not an independent variable. It is whatever value is required to deliver that amperage under the actual conditions of anode-to-cathode distance and solution conductivity. Relatively small parts usually means relatively close anode-to-cathode spacing and relatively low voltage. A 9-volt rectifier is probably safe if parts remain small and anode-to-cathode distance remains short. But it is better to find someone who is doing a similar job and doing it in a similar way, and find out from them what voltage they have found necessary than to try to guess :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello sir again
How to hard chrome plate inner surface of guns barrels? I am using lead antimonial rod coated with oxide in inner side of guns barrels -- is this okay or not?
How many ampere and volt rectifier will be used if the distance of anode and cathode is 0.5 inch and cathode surface area is 18 square inch to 40 square inch?



For small guns barrel (9 sq inch) is working but not for large (28.8 sq inch).
aziz ullah [returning]- peshawar Pakistan
A. Hi again, Aziz. I have no personal experience in chrome plating of gun barrels and can only report 'book knowledge'. Guffie says 2-4 Amps per square inch, which would mean 18-36 Amps for the small gun barrel and 57-115 Amps for the large. He also suggests that while 9 volts is usually enough, 12 volts is rarely not worth it.
But what he also makes a big point of is that the anode area should be as large as possible or the anodes will passivate and simply stop plating. That sounds like maybe that's a problem for you on the larger barrels.
Peger is quite specific: 4.5 volts if anode-to-cathode spacing is 1/2 inch.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Help me in the following problems:
Temperature requirement for hard chrome?
and the relation between current and temperature?
Why does the surface become dull not shiny?
- peshawar Pakistan
May 31, 2021
by Weiner & Walmsley

on eBay (rarely)
or Amazon (rarely)
or AbeBooks (rarely)
(affil link)
A. Hi Aziz. The most common hard chrome plating temperatures is probably 130 °F / 54-55 °C., but Peger says 140 °F.
The allowable current density increases at higher temperature, but unfortunately the efficiency decreases, so changing the temperature isn't usually much of a strategy. Weiner & Walmsley has dozens of graphs covering the interaction of all the variables if you can find it in a library. The "bright plating range" is narrow.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. We are using constant voltage rectifier for hard chrome plating with the output current capacity of 8000 Amps; however, the current creeps up causing extra chrome being deposited onto the surface.

Any pointers on what could be the possible cause?
Hassan Bin Mazhar- Bury, Manchester, England
October 11, 2021
A. Hi Hassan. Are you using only tank anodes? If so, my first suspicion would be that something is wrong with the rectifier or its gauges.
But A = V / R, and the bulk of that R is solution resistance across the gap from anode to cathode. So I suppose if you are using conforming anode surfaces very close to your component it's vaguely possible that as the surface of the plating grows closer to the anodes the resistance is being reduced by enough to affect the current.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Thanks a lot for the reply Ted. We did check our rectifiers and turns out they are not stabilizing the voltage efficiently and there is a lot of ripple.
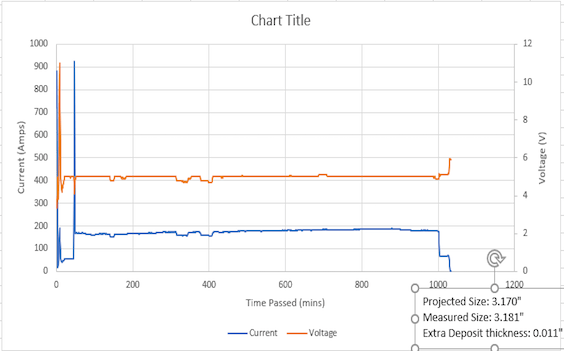
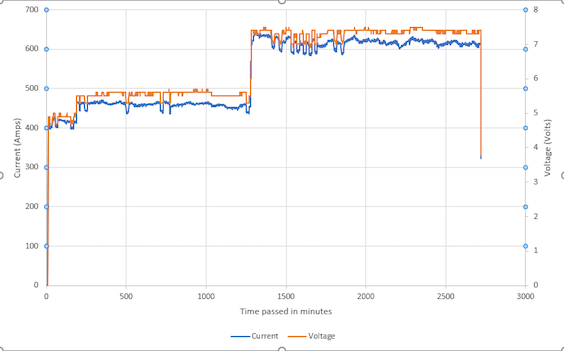
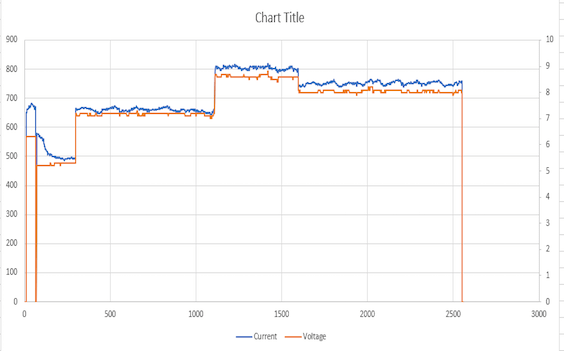
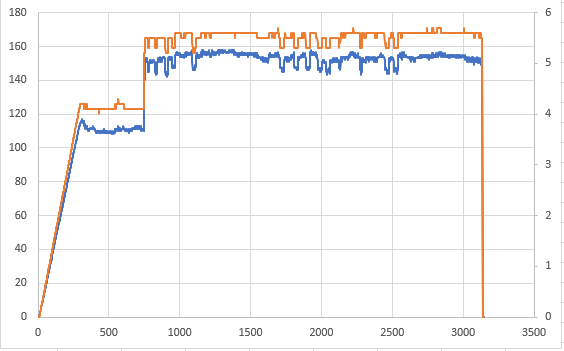
Are there any dampers that we can use to stabilize the output from the rectifiers?
Hassan Bin Mazhar [returning]- Bury Manchester, England
A. Hi again, Hassan. Yes, external devices have been used on plating rectifiers to stabilize the voltage for decades. But don't use the word "damper" in your search, do a google search for "filter chokes for plating rectifier ripple"
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello,
I am an employee of a cylinder fabrication and repair factory.
We have 2 chrome baths:
• new one we are using 8000 Amp rectifier.
The first issue on new one is that when I set the current (for example to 1000 Amps) its withdrawal more the 2800 amps (using 25 current density and try to keep temperature on 52 °C).
Another issue is that the chrome layer is reaching 20 microns in only 10 minutes which is too high compared to the old one.
I have the option to use voltage control on the device. Can I use that and which formula can be used?
employee - jeddah, Saudi Arabia
December 21, 2021
A. Hi Ammar. I don't fully understand what you are describing, but if you are using the same chrome solution in both tanks, one is not plating much faster at the same amperage ... it sounds like your rectifier or meters (or the way they are installed) is wrong.
Yes you can "plate by voltage", many people recommend it, but the required voltage will depend upon the anode-to-cathode distance, which may be different for different size cylinders.
Please read the rest of this thread, then get back to us with more detailed questions.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Mr.Ted Mooney, P.E. RET
Appreciate your swift reply.
I have two rectifiers, one is old - other is new with thyristor type. My issue whenever I set to desired amperes still shown on screen more [for example I have Rod dia 90 L:1400 A/dc I use 30 A/dm^2 results is about 990 Amps - bath temperature is 50-52 °C - when starts THE RECTIFIER IS TAKES MORE THE 2700 Amps] and platings gives 20 micron in 10 minutes - the FINISH IS NOT SHINING AT ALL its milky color
employee - jeddah, Saudi Arabia
A. Hi Ammar. I don't know what else can be said :-)
You already knew the amperage was too high; but it's confirmed as too high a second time from thickness measurements because it's plating too fast, and you know it's too high a third time because you are seeing 'burning' instead of shiny chrome. We can't troubleshoot your rectifier from here but it's apparently the problem.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.

Q. Sir
I am having some difficulties on my chrome plating plants -- today I have noticed that the chrome does not hold/stick on the rod at all.
employee - jeddah, Saudi Arabia
Q. Hello Sir,
thanks.
I am using formula L x D x 3.14 x 25 as current density, so for the chrome rod I need to plate is 900 Amps with temperature 52-56 °C degrees - voltage is fixed to 15 V.
Am using ampere control.
The chrome chemical test results are:
1. Chrome Acid 104.4 g/l
2. Ferric content 4.12 g/l
3. Density 16 baume [1.13 g/cm^3]
I did add the cleomechromic acid to reach the standard 280 g/l, am not adding catalyst.
employee - jeddah, Saudi Arabia
A. Hi again, Ammar. I apologize but I just can't understand your 4 postings. You were trying to chrome plate with a chromic acid concentration of 104.4 g/l when you know it's supposed to be 280 g/l? Are you trying to chrome plate without catalyst at all (not possible), or are you saying it's now at 104.4/280 of what it should be? You believe that your rectifier meter readings are wrong but you are using them anyway? I'm sorry but we're just not communicating. Sorry, but I can't understand your situation, or you can't understand what I've been trying to say, or both :-(
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.

Q. At the shop we do hard chrome plating plunger. When we have a problem the plunger we plating results in a broken or burnt. What is the cause?
Azwadi RohansyahShop employee - Palembang, Indonesia
January 14, 2022
A. In the photo, the part is after immediately after chrome coating (why is the insulation so clean from below), or after working in some kind of unit? Please describe the problem in more detail. The composition of the bath, application modes preparation.
Nik Erm- Nizhniy Novgorod, Russia
The insulation looks clean because after the chrome coating we flush it with water.



The process is as follows :
Etching ; Current Density 30 A/dm2
Ampere 4500 A
Temperature 51 °C
Time 40 s
Cr Plating ; Current Density 30 A/dm2
Ampere 4500 A
Temperature 51 ° ~ 53° C
Time 15 Hours
Before hard chrome, the workpiece is ground first.
Plating tank shape : 1000 W x 2000 L x 2000H
Plating solution qty : 1000 x 2000x 1800 = 3600 litres,
chromic acid input : 900 kg & sulfuric acid input : 9 kg
Shop employee - Palembang, Indonesia
A. A visual inspection of the provided blanks revealed non-coverage in areas with low current density. On the protruding parts (high current density areas) of the part, shiny chrome is deposited. At the same time, roughness is present on some of the surfaces where chromium has deposited.
This defect is known and its occurrence usually occurs when the covering power of the chromium plating electrolyte decreases or when the current source (if the current source itself is in good condition) cannot maintain the specified current due to the high resistance of the electrolyte.
A decrease in the covering power of the electrolyte is possible due to its contamination with iron ions, trivalent chromium, etc., with an increased content of sulfuric acid or a low content of chromic anhydride.
Also, ion contamination with ions of iron, trivalent chromium, etc. increases the resistance of the electrolyte and maintaining a given current (30A/dm2) requires more voltage than the current source can produce. For example, a current source can deliver 12 volts, and in order to maintain the desired current (30A / dm2), with a high electrolyte resistance, 15 volts are required, since the current source cannot give them out, it will reduce the current (for example, to 15 A / dm2) and the blanks will not be covered.
If you have a Hull Cell
⇦ huh?
(you can make it yourself), then the problem can be quickly identified and subsequently eliminated.
If the hiding power is reduced due to an increased content of sulfuric acid, then it can be precipitated with barium carbonate by determining its required amount from the result of the analysis or by selecting this amount using the Hull cell.
Sulfuric acid is precipitated with barium carbonate according to the reaction H2SO4 + BaCO3 = H2O + CO2 + BaSO4,
based on 1 g of sulfuric acid, introduce 2.012 g of barium carbonate.
If the hiding power is reduced as a result of the increased content of trivalent chromium, then the electrolyte needs to be worked out.
Working out should be carried out with an anode area 5-10 times larger than the cathode one (the part for working out should have an area ten times smaller than the anode area, but not less than 10 dm2). The study must be carried out with periodic stirring of the electrolyte so that trivalent chromium ions are constantly present in the anode layer. Current strength 10 -30 A / dm2. The duration of study is 24-72 hours (depending on the amount of trivalent chromium accumulated in the electrolyte). Efficiency should be controlled according to the readings of the voltmeter; in the process of working out, a gradual decrease in voltage should occur. This measure will increase the covering power and reduce the stress on the bath.
If the hiding power is reduced as a result of contamination with iron or copper ions, then a reduction in its content is required. Of the possible ways:
Diluting the electrolyte by 2 times with water and adding chromic anhydride there (the effectiveness of this method is first checked by the Hull cell).
Addition of complexing agents that bind iron and copper ions into complexes, which are partially deposited on the bottom of the chromium plating tank, and partially leave them in the electrolyte in complexes that do not affect the course of the chromium plating process
The use of special membrane plants that allow you to clean the electrolyte.
To determine the correct solution to the problem, first of all, it is necessary to conduct electrolyte studies on the Hull cell.
- Nizhniy Novgorod, Russia
March 25, 2022
No dead threads!
Your Q, A, or Comment puts this thread on The Finishing.com HOTLINE.
