
Curated with aloha by
Ted Mooney, P.E. RET


Faraday's Law of Electrolysis
The Simple Relationship which Faraday Discovered Makes Chemistry & Electrochemistry Much Easier!
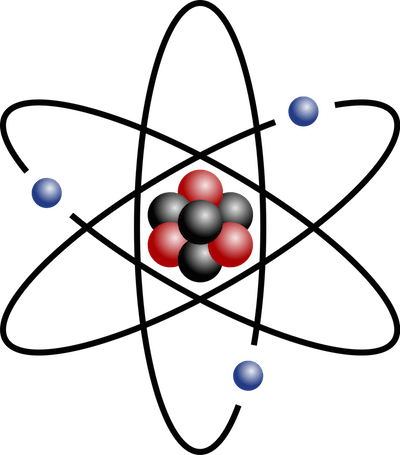
You probably know that elements are made of very tiny atoms, which in turn consist of a nucleus at the center with a number of protons and neutrons glued together, and a number of electrons orbiting the nucleus.
(credit: Wikipedia)
Each proton has a positive charge, each electron has a negative charge ... and every atom has as many electrons as protons for a resultant electrical charge of zero. The number of protons in the nucleus of the atom is called the element's "atomic number" and determines what the element is. If it has one proton it is Hydrogen (H) and has an atomic number of 1; if it has two protons it is Helium (He) with atomic number 2, if it has three (as in the above picture) it is Lithium (Li). If it has four it's Beryllium (Be), five it's Boron (B), six it's Carbon (C), seven it's Nitrogen (N), eight it's Oxygen (O), nine it's Fluorine (F), ten it's Neon (Ne) ... on up through the 92 protons of Uranium (U).
Neutrons have no charge, and the number of neutrons isn't nearly as important as the number of protons. Neutrons and protons are roughly the same weight and electrons are of almost negligible weight. Most atoms have a few more neutrons than protons, so the "atomic weight" of many elements (the weight of one atom of the material) is usually a little more than twice it atomic number. If two atoms have the same number of protons but a differing number of neutrons, we call the two "isotopes" of each other -- most properties of two isotopes of the same element are the same, but there are some differences, such as (obviously) their differing atomic weights.
Electrons don't orbit the nucleus in ring-like paths though; their paths are more like an egg shell, a ping pong ball, or a thin cloud the shape of a sphere -- electrons are said to occupy concentric "shells" around the nucleus.
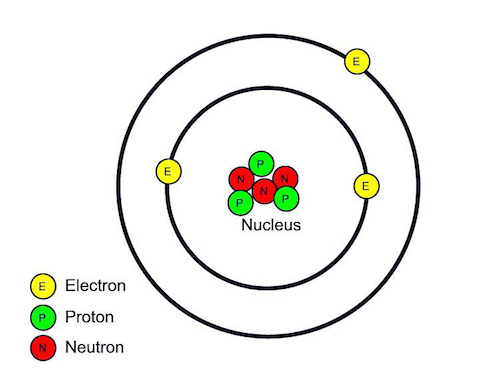
(Credit: http://girlscientistmagazine.blogspot.com)
But each electron doesn't get its own shell; rather, those concentric shell spaces have room for up to a certain number of electrons. Nature is complicated and no one truly understands, so don't despair that you don't understand why, but the innermost shell has room for 2 electrons, and the next concentric shell has enough room for 8 more (after that it gets too complicated for most of us to bother with) 🙂
This issue of electrons shells having "enough room for" is what drives chemical reactions, what chemistry is all about, so bear with us, as we start back with element no. 1, Hydrogen, again -- noting that it has only one electron, but its electron shell has room for two. Then Helium with 2 electrons has no more room for another electron in its shell. Lithium has three electrons, so the first two fill the inner shell, and the third starts that second shell that has room for eight. Thus it continues up through element no. 9, Fluorine with two of its electrons filling the inner shell, and its other seven nearly filling the next shell; and element 10, Neon, with 10 electrons, 2 of them filling the inner shell space, and the other eight filling the second shell.
Now, picture a wheel for a truck, with provision for eight lug nuts (like that second electron shell with its room for eight electrons). The wheel is balanced if all eight lug nuts are present, but is out of balance if any are missing. It is this difference between the "room" for electrons in the shells and the actual number present in them which drives chemical reactions.

(Credit: http://trucktrend.com)
You can hopefully at least understand, if not quite envision, an atom of lithium with it's three electrons, one of which is in the outer shell, and an atom of fluorine with it's seven electrons in the outer shell, getting together as Lithium Fluoride (LF) and "sharing" their electrons so we have a total of eight in the outer shell for "balance". (As shorthand for "the number of electrons in the outer shell of a lithium atom is 1" we say that the "valence" of lithium is +1. Fluorine has 7 outer shell electrons, so it is lacking one of the eight it would need for balance; rather than saying that the valence of fluorine is +7, it is more descriptive & accurate to describe its valence as -1.
Two lithium atoms of valence +1 could get together with an oxygen atom of valence -2 to form Li2O with a total of eight electrons in the outer shell; or three lithium atoms could combine with nitrogen of valence -3 to form Li3N.
Although we can't accurately predict every chemical reaction from this alone, especially as we move on to more electron shells with different numbers of electrons and the more complex way they are filled and balanced, it does go a long way. For example, we can predict boron nitride (BN) from boron's 3 outer shell electrons and nitrogen's 5, and that Neon with its full shell of 8 electrons is balanced and "inert" and will not combine with other materials, and that Helium with its full inner shell of two electrons, and no outer shell in also "inert" and will not participate in chemical reactions.
Moving on to Electroplating
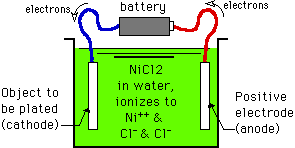
A commonly plated metal, Silver (atomic number 47, i.e., 47 protons), has one electron in its active shell (valence +1). Also commonly plated are Nickel (atomic number 28) and Zinc (atomic number 30) each with two electrons in their active shell (valence +2). Chrome (atomic number 24) acts as if it had six electrons in its active shell, for a valence of +6 (although the electron shell oddities may actually be a bit more complicated).
If we have a plating solution based on nickel, like nickel chloride (NiCl2), and we put a battery between two nickel electrodes immersed in that solution, the battery will pump electrons from its positive pole (which we call the anode) to its negative pole (called the cathode). As two electrons are stripped away from the anode and flow through the wiring to the cathode, the nickel atom can't remain an atom with balanced proton and electron charges, but becomes a positively charged nickel "ion" (Ni++) which dissolves into the NiCl2 solution. At the same time, the cathode now has two extra electrons, so when a Ni++ ion approaches it, the electrons will merge with the ion to re-form a nickel atom. Thus, moving two electrons from anode to cathode via the battery causes one nickel atom to dissolve from the anode and plate out onto the cathode.
If you are plating silver, which has only one electron in its active shell, valence +1, then pumping one electron from anode to cathode will cause one atom to ionize from the anode, migrate through the solution, and deposit onto the cathode. If you are plating chrome with its valence of +6, you'll need to move 6 electrons from anode to cathode to move one atom.
Gram Atomic Weight
We previously mentioned that because there are usually a few more neutrons than protons in an atom's nucleus, the atomic weight (AW) of elements is usually a little over twice their atomic number (AN). Thus
Silver: AN=47, AW=107.87
Nickel: AN=28, AW=58.69
Zinc: AN=30, AW=65.38
Chrome: AN=24, AW=52.01
A gram atomic weight, or gram molecular weight, or mole is simply the atomic weight expressed in grams. Thus, one gram atomic weight of silver is 107.87 grams; one mole of nickel is 58.69 grams. (For those who are advanced enough in chemistry to have heard of Avogadro's number, one gram atomic weight or mole of an element also always contains 6.02 x 1023 atoms).
Faraday's Observation
Faraday's Law relates the electrical current flow to how much metal will be deposited.
For all metals 96,485 ampere-seconds (i.e., a current of 96485 amperes flowing for one second, or 1 ampere flowing for 96,485 seconds, or any current and amount of time which multiplies out to 96,485) will deposit 1 gram atomic weight of the metal divided by its valence. Thus 96,485 ampere-seconds will deposit 107.87 grams of silver, or 58.59/2 = 29.30 grams of nickel, or 65.38/2 = 32.69 grams of zinc, or 52.01/6 = 8.67 grams of chrome. This quantity of 96,485 ampere-seconds is sometimes called "a Faraday" or the Faraday Constant.
A possible complication is that some metals oxidize to different valences under different conditions. Copper, for example, despite one electron in its active shell, exhibits a valence of +2 in acidic solutions like copper sulphate. Tin works at a valence of +2 in acids, but +4 in alkaline solutions. Gold ions can have a valence of +1 or +3.
(affil links)

free pdf is currently available from academia.edu
With this knowledge you know how much metal will be deposited based on the current & time, but if you find the calculations tiring, the Metal Finishing Guidebook has a great chart (page 812 ^ page 880 in 2013/2014 edition) which does all the math for us, giving us the number of ampere-hours required to deposit a plating of 0.001" thickness of the various metals over an area of 1 square foot, which you can scale up or down for different thicknesses or surface areas.
Efficiency
We simplified just a little bit in claiming that Faraday said that 96,485 ampere-seconds will deposit 1 gram equivalent weight divided by the valence ... Yes, it will always drive that much electrochemical activity, but all 100% of that activity may not go to what we'd like it to go to. Some portion may liberate hydrogen gas from water rather than laying down metal.
Suppose for example that you instantaneously apply a huge current to an electroplating cell. The electrons will move from the anode to the cathode at the speed of light, but the nickel can't possibly dissolve from the anode instantly, the ions migrate over to the cathode instantly, and plate out instantly. Rather, that huge surfeit of electrons must be satisfied in some other way, and what will actually happen is that those excess electrons will pull hydrogen out of the water at the cathode and evolve it as hydrogen gas. In actuality, the efficiency of some plating processes approaches 100% if done at reasonable current flows, but the efficiency of some other plating processes like chrome plating might never even hit 25%.
Whenever we try to plate too fast, too much hydrogen evolves, and/or the plating does not have enough time to build a proper crystal structure ... and the deposit is discolored, frosty, or non-adherent -- a situation which we call "burning".
Voltage
As explained, it is ampere-seconds -- current x time -- which does the electroplating, not voltage. The voltage to be applied is simply whatever is needed per Ohm's Law (Voltage = Amperage x Resistance ) to make the current flow at the correct rate.
Empirical Knowledge
Although we understand some principles like Faraday's Law and Ohm's Law very well, we do not have sufficient knowledge to understand from theory and first principles how much amperage or voltage we can use without burning -- that is currently just empirical knowledge. We know, for example, that bright nickel plating should usually be done at about 40 amps per square foot of surface area; but we know it because we've tried it and at too low a current density it's not bright, and at too high it burns -- not because we truly understand all there is to know about ion migration and field gradients, boundary layer mechanics, etc.
Please Share
Good luck. Please feel free to share this page, and if you have suggestions for improvement to it, please email mooney@finishing.com.