
-----
What is proper Rhodium Plating Thickness and how to measure it?
Q. I am working with a jewelry company as production In charge. We export to US and middle east market. One of my customers has asked us to specify the thickness of Rhodium plating that we are giving to white gold articles. I have a rough estimate of 0.5 micron but cannot say exactly the thickness of plating. How to measure the thickness of microplating? To what extent can the thickness of rhodium plating be increased?
Dinesh Kumar SinhaJewellery manufacturer - Mumbai, Maharashtra, India
October 25, 2008
A. Hi, Dinesh. 0.75 to 1.0 micron should be practical if you are looking for thicker plating. Considering the very high cost of rhodium, good instrumentation such as x-ray fluorescence should be justifiable.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Hi Dinesh,
You may measure the thickness as Ted said.
Usually for plating precious metals like gold, platinum, Palladium, Rhodium etc. it is always good to know the wt. in mg deposited on 1 dm square, for one micron. By this you can be sure how much worth in rupees of metal is deposited. This can also be considered as countercheck. For gold it is 178 mg/dm2/micron, Palladium it is 90 mg/dm2/micron and Rhodium, I understand deposits 40 mg/dm square to give one micron. If I am not confusing you, I will give a calculation as below:
Generally Rhodium is deposited @ 6-8 mg/amp-minute @ about 2 grm/ltr Rh in the bath.So if you back calculate you can get the time required to get 40 mg/dm2 and that will be equal to 1 micron.
Thus you can fix rate for the thickness to the customer with ease.
Best of luck.

T.K. Mohan
plating process supplier - Mumbai, India
January 6, 2009
Q. We are a manufacturer of high quality jewelry based in Gujrat India. We have been asked by our client to deposit between 3 to 5 mils thickness of rhodium on our jewelry products. We have always deposited rhodium in milligrams. Can you please advise me how many milligrams of rhodium is equal to 3 to 5 mils?
Thanking you in advance
PROPRIETOR - NAVSARI, GUJRAT, India
April 8, 2011
A. Mils in this context is a slang term being used by some and they usually mean millionths of an inch. They probably want 0.1 micron thickness of rhodium, and milligrams is a weight and will not tell you the thickness of your plating unless you also know the surface area.
Neil BellAlbuquerque, New Mexico
Q. My company sells high quality costume jewelry. When I wanted to know the micron quantity of the products from one of our producers, he told me they plate it with "3U rhodium". I never heard this unit before, can anyone help me?
Theresa Berger- Vienna, AUSTRIA
September 20, 2012
A. Maybe his "U" means "µ" = microns.
But 3µ thickness is very high for rhodium plating. You can have problems of cracks with such an high thickness.
Best regards ;)
- France
September 22, 2012
A. Servus, Gruss Di, und Hallo Theresa.
In my experience plating costume jewellery for Nina Ricci we found plating rhodium over gold to ascertain minimum thickness proved to be 0.000001" (at this thickness no gold colour was present). We decided to plate 0.000003" rhodium minimum to ensure quality. The gold thicknesses were 0.000008-0.00001 as a norm. I believe the "U" is designation for MICRO/MILLIONTHS of an inch. This would be 0.077 microns. For costume jewellery this would be enough to ensure the items retain their brilliance under normal wear (no abrasion, mechanical or chemical).
Servus!
- Toronto Canada
September 28, 2012
March 22, 2013
Q. Hello,
I wonder if someone can help me over here.
I am looking to manufacture my jewellery collection and very confused regarding the following.
What base metal should we use to achieve quality? Copper or Alloy.
Specifically we want to use 18k White gold plating. Over here, what would you recommended the thickness of the plating to be? Someone suggested 0.03 µm or 0.1 µm.
Quality is very important to me and your suggestions are as well :)
Many thanks,
Gee
- London, England
A. Hi Gaurav. I could be misreading your inquiry, but I'm not confident that white gold (8N) plating is really what you are looking for. May I request that you review our FAQ, "Rhodium Plating and White Gold" and then clarify if you still are looking for white gold plating? Eric's posting right above yours gives some good info on thickness of the plating. Thanks.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
June 12, 2014
Q. Dear Sir,
Currently we are manufacturing silver jewellery and we do rhodium for all our jewellery as it is required by our customer.
How do we measure the rhodium plating thickness manually?
How do we measure the silver plating and silver anti tarnish coating also manually?
Please advise what is the calculation for this procedure.
regards,
Sundaram
JEWELLERY MANUFACTURING - JAIPUR India
A. Hi Sundaram. I think there is little possibility of measuring the thickness of rhodium anti-tarnish plating on silver costume jewelry "manually" -- which I interpret to mean with a micrometer or like instrument. And viewing it by metallographic cross-section sounds quite unlikely unless you run a world-class lab.
It is probably possible to track thickness with an x-ray fluorescence machine, but these are expensive, so suitable for manufacturers but not for custom jewelry designers. The most practical technique is probably as described by TK Mohan; for background:
Metals are electrodeposited in proportion to the applied ampere-seconds, as discovered by Faraday with his Law of Electrolysis. 96,485 ampere-seconds (coulombs) deposits exactly one gram equivalent weight of metal if the bath is operating at 100% efficiency. From the valence state and atomic weight, you can determine exactly how much weight you have deposited; and from the density of the metal, what volume you have deposited. Dividing by the surface area of the work, you can determine the thickness. All of this conversion effort can be saved by going to the "Electrochemical Equivalents" appendix in the Metal Finishing Guidebook, which incorporates all those conversion factors for you and offers the answer that it takes 22.9 ampere-hours per square foot to deposit 0.001" of rhodium and 6.2 ampere-hours to deposit 0.001" of silver.
But most plating baths do not operate at 100% efficiency. In this case Faraday's Law of Electrolysis still holds but a portion of the ampere-seconds are going towards liberating hydrogen from the water of the plating solution instead of depositing silver or rhodium. TK suggests that you can determine the efficiency that you are operating at, or simply incorporate the efficiency into your calculations, by measuring how much rhodium you deposit once (or periodically), and then know how much rhodium you are depositing on your jewelry by simply applying a scaling factor to your ampere-seconds measurement. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
June 2014
Q. Hi,
I am starting a Jewelry line that with brass base metal and rhodium plating. I have been advised to use 0.075 microns of rhodium plating on my jewelry, and that this will last up to 1 year with normal wear.
Is this a normal thickness to use, or should I go thicker?
What are companies like Swarovski's jewelry plated at? Similar?
Thanks in advance,
James
- Toronto, ontario, Canada
August 21, 2015
A. Hi James. Per an ad on Amazon, Swarovski electroplating is "3MIL". They claim that over 90% of jewelry suppliers plate 3X thinner.
To electroplaters, 3 mil means three thousandths of an inch but, as Neil notes above, jewelry manufacturers mean 3 millionths of an inch, not 3 thousandths, and that's quite close to your 0.075 microns. Even at that, it's pretty thin: a pair of standard dice cubes of plating metal would cover about two thousand square feet of jewelry :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
August 2015
August 24, 2015
A. Good day James.
Rhodium @ 0.075 microns = 2.55 millionths of an inch is very good for pins, brooches, and items which do not receive any mechanical wear.
A bracelet or a ring or a pendant will not have the same "wear ability", due to mechanical abrasion factors, and acidity of the skin reacting with the base metal/substrate.
I do not know your process, but brass/copper alloys as substrates for jewelry to be rhodium plated, must be given a hard, lustrous barrier coat of metal to produce a shiny, hard surface to retain the quality and brilliance of rhodium plating.
In the past, acid copper and nickel plating was predominantly used prior to rhodium.
Nickel plating does pose health risks, (regarding dermatology) as it is banned in Europe.
A barrier plating of palladium (or other metal) instead of nickel is often used prior to rhodium.
Hope this is helpful and good luck with your endeavor.
Regards,
Lab Tech. - Whitby, On, Canada
Q. If we are wanting to produce some high end sterling silver jewellery (925) and want a good luster, finishing and durability of our product. Then what could be the best thickness of rhodium plating. We are also wanting to know the rhodium plating procedure and thickness used in Bangkok 925 sterling silver jewelry.
Regards,
- Panipat, Haryana, India.
June 29, 2017
A. Hi Arun. You can ask Bangkok 925 what thickness of rhodium they apply and their procedures; they may tell you, although I think they might keep certain steps confidential. You can also test it to find the thickness. But to post responses about what one particular supplier does could drift towards assisting in industrial espionage, and sorry but we don't have time to carefully audit such a discussion :-)
Courtesy of Metal Arts Specialties, we have a detailed article about rhodium plating of jewelry for download in our on-line library here; although it is actually directed at rhodium plating on white gold, it should answer your questions. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
July 2017
A. The test that Ted mentions is usually done by X-ray fluorescence and our experience is that 0.1 micron to 0.2 micron of rhodium will provide good wear resistance on sterling silver jewelry.
Neil BellAlbuquerque, New Mexico
July 20, 2017
Q. Hi, we supply silver jewellery items which need to sometimes be bought in bulk and stored by our customer for 2-3 years. We need these to maintain a silver finish and not tarnish and so have requested that all of our items are rhodium plated by our supplier BUT items are being returned because they have tarnished and our supplier is saying that they are only able to give us a 6 month warranty on the rhodium plating. This is not satisfactory for us or our customers .
Can you suggest what has happened or what we can do to achieve our requirements please?
- Sydney Australia
September 4, 2018
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. Hi Christine. The first thing is that you as the supplier, perhaps in concert with one or more platers, must specify the plating process. Simply saying 'rhodium plated' is not enough: you can see in the discussion above the relentless pressure to always reduce thickness and cost. You need to write an actual specification for the plating, and the plater must certify their compliance to it.
Secondly, nothing lasts forever, but storage conditions have a great deal to do with how long they last. If components are going to be in storage for 2-3 years, prescribed storage methods probably must be instituted. Perhaps storage in sealed plastic bags with desiccant like over-the-counter medicines. Perhaps VCIs (volatile corrosion inhibitors) can be employed if parts can't remain sealed, the cloth on storage boxes for silverware is sometimes infused with VCIs.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
September 2018
A. It is next to impossible to production plate crack free rhodium, and silver will migrate right thru the cracks. Also, if the rhodium is thin, silver will come right thru it anyway. Put a palladium barrier between the silver and rhodium.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

September 6, 2018
Q. Myself having a plating shop would like to know how much rhodium will be deposited on a pendant with size 50 mm X 50 mm
Suresh SharmaRhodium - Aurangabad, India
November 9, 2019
A. Hi Suresh. People are usually helpful here, but it's probably not practical for them to do the homework. You need to say what the base metal is, whether there will be a white bronze or nickel underlayer, why you're rhodium plating the pendant, and what durability/price range you expect...
For example, if the pendant is silver, people usually rhodium plate it just thick enough to deter tarnish for the display period (many people like real silver, and like the fact that it tarnishes, but they don't want to buy dirty looking jewelry which looks like it was previously worn by someone else). On the other hand, if you are rhodium plating white gold, the pendant will be much more expensive, and most white gold is not white enough to look good after the rhodium wears away, so you'll need a more durable rhodium layer. A silver pendant might need only 0.05 micron thickness; whereas a very high quality gold one might use 0.5 microns.
Courtesy of artisanplating.com, we have a great powerpoint presentation available which covers all these issues .
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 2019
Rhodium thickness?
Q. I have jewellery piece with area 20000 dm square. How much rhodium will get deposited on piece with current 1 amp.
The plating is done for 120 seconds.
Please let me know how much rhodium will get deposited.
employee - Mumbai
November 11, 2019
A. Hi Suresh. Your jewelry piece is surely not 20000 dm2 (2000 square feet). Maybe you mean 20000 mm2 (31 square inches)?
Per the 'Electrochemical Equivalents' chart in the Metal Finishing Guidebook, at 100% efficiency it requires 22.9 Amp-hours to deposit 0.001" thickness on one square foot. Which means it would require 22.9 X 31/144 or 4.93 Amp-hours to deposit 0.001" thickness on 31 square inches. If you plated for 1 amp-hour rather than 4.93 Amp-hours, the plating thickness would be 0.001" / 4.93 = 0.0002". You are plating for 2 Amp-minutes, which is 1/30 of an amp-hour, so the thickness would be 0.0002" / 30 or 0.00000676" or 0.17 microns.
However, rhodium plating efficiency is extremely variable with efficiencies as high as 60-80% reportedly possible with some proprietary solutions operating at maximum concentration and ideal conditions, but less than 10% in many actual applications ... meaning that your thickness might be as high as 80% of 0.17 microns (quite unlikely) or as low as 10% of 0.17 microns (or even less). The short answer is that such calculations won't really help you until you have some experience in measuring the rhodium plating thickness you achieve, but the thickness is probably not enough for practical application :-(
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 2019
How much gold thickness equals 0.25µm rhodium thickness?
Q. Hi there,
I'm purchasing sterling silver jewelry directly from a manufacturer. The manufacturer uses only 0.025 µm for rhodium plating. How thick should our gold plating be to achieve the same quality as the rhodium plating?
Also, do manufacturers polish sterling silver before plating it?
Best
Charlie
- South San Francisco, California
October 8, 2020
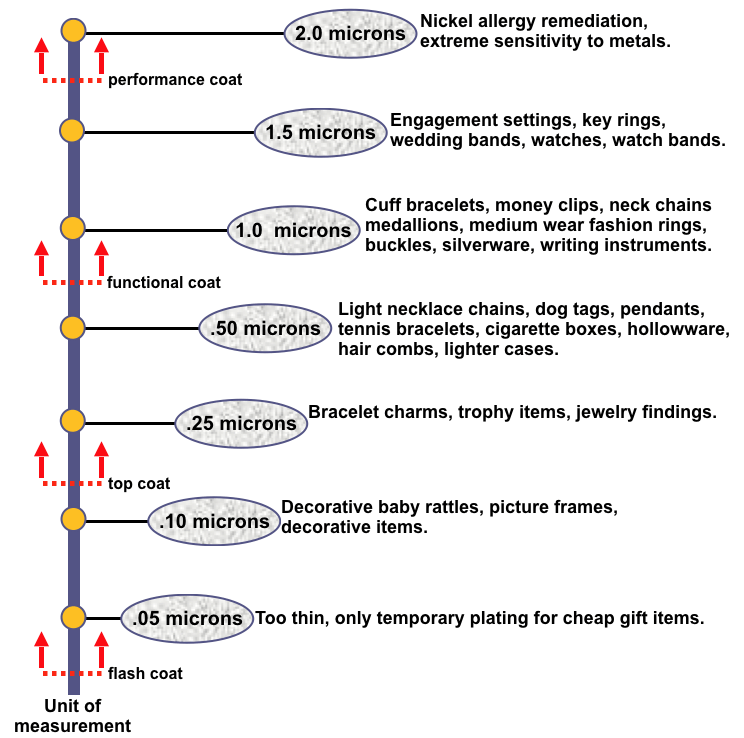
A. Hi Charlie. Rhodium plating on Sterling Silver is done primarily to reduce tarnishing, not to change the look of the jewelry, and at 0.025 µm it is probably essentially invisible. This chart, courtesy of David Vinson of Metal Arts Specialties (artisanplating.com), reinforces that idea by starting at twice that thickness for the low end of "too thin" -->
But you would not be applying gold plating to reduce tarnishing, but to offer the jewelry a gold color, so unfortunately your question isn't really answerable. My understanding is that you need about 0.15 to 0.2 µm of gold to exhibit a good gold color, so that may be the answer you are seeking.
For comparison, super premium watches are gold plated with about 7 microns, very high quality vermeil jewelry is 2-1/2 microns of gold, "good" quality gold plated jewelry is sometimes called micron jewelry because it is plated to about 1 micron. Of course lots of jewelry items are plated significantly thinner than 1 micron, down to probably this 0.15 micron. But again, if the rhodium plating is too thin or is even absent, people might not even know it, whereas if the gold color wears off in one or two days, you're not going to have happy customers :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread

