
-----
How to calculate/measure the thickness of zinc coatings
Q. Hi Everyone.
I have read some of the threads and there is clearly a wealth of knowledge here.
I have a dilemma, I would like to know, is there a way I can use the readings of an Elcometer in Microns to calculate the coating thickness of zinc plated sheets (Z275), Aluzinc (AZ 120) and Magnellis/ZAM (AZM 80)
I would greatly appreciate your feedback and even reference to supporting articles to use if needed.
Flat steel product specialist - South Africa
June 7, 2024
combo magnetic & eddy-current coating thickness tester

on eBay or Amazon
(affil link)
A. Hi Brian.
I'm not fully confident that I exactly understand the question, and Elcometer makes a few different types of instruments and probes, but ...
A gauge with a magnetic force probe, and which is calibrated, should give accurate thickness readings for any non-magnetic coating, including the ones you mentioned -- but an eddy current device (designed for coatings on non-magnetic substrates) does not sound like a good choice.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Yes. You could use an Elcometer (that's a brand of many instruments) that is typically used in a galvanizing plant to measure zinc thickness on steel, to measure the thickness of the named other coatings.
Most galvanizers use a magnetic force instrument (Elcometer is one brand of several), to measure the non ferrous coating on a ferrous substrate.
If you want to measure thickness of say paint on top of galvanizing on top of steel, then there are instruments that use both eddy current and magnetic technique and can give the thickness of each coating.

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

June 21, 2024
A. My friend Mike Rometer says he can measure the thickness of plating on rod stock or flat stock without using any fancy instruments.

Tom Rochester
CTO - Jackson, Michigan, USA
Plating Systems & Technologies, Inc.

July 18, 2024
A. Yes, Mike is very direct with his answers.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I have purchased galvanized wire. Thickness of wire is 2.50 mm and zinc coating thickness measurement by microscopic method is 7.00 micron. Then how much GSM for this thickness?
Mukesh Patel- India
January 17, 2025
But7 micron coatings are quite then for galvanizing; I'd think it might be zinc electroplating at those thicknesses/
(this entry appended to this thread by editor in lieu of spawning a duplicative thread)
Zinc coating verification on a steel sheet
Q. Hello everyone.
I've read some threads, and there's certainly a lot of information on this subject.
I have a dilemma: I'd like to know if there's a way to use the readings from a magnetic meter (ININIPA brand) in microns to calculate the coating thickness of galvanized sheets (Z100).
The way I'm currently doing it would be this way, but I'm not entirely sure:
- A galvanized sheet with a thickness of 0.54 mm and a coating according to its original technical data sheet is Z100.
- To confirm that the coating is really Z100, I take the sample with the magnetic meter and multiply it by the zinc density to give me the coating thickness, which in this case is Z100.
The result was:
15 (magnetic meter result). I multiply it by the density of zinc, and it would be:
15*7138 = 107 (Z107 would then be your actual result).
I would greatly appreciate your comments and even references to supporting articles if needed
empleado de oficina - Paraguay-Asunción
September 2, 2025
A. Hi,
For the record: due to contaminants, porosity, pinholing and so forth, ISO 2081 says to use 7.1.g/cc for electroplated zinc.
If you use that that galvanized zinc, your calculated thickness would be one-half of 1% lower, which is inconsequential considering the limits to precision that you're working with 🙂
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Other options include weigh-strip-weigh and/or using a micrometer. Keep in mind that these tests measure both sides!

Tom Rochester
CTO - Jackson, Michigan, USA
Plating Systems & Technologies, Inc.

⇩ Related postings, oldest first ⇩
Q. I would like to know what will be the thickness of the electroplating in microns, on MS sheets of flats, when 610 gms/sqmt of zinc is used.
Vinod KumarDesign engineer - India
August 14, 2008
A. Hi, Vinod. Usually electroplating is expressed in thickness but phosphatizing is expressed in weight because the meaning and usefulness of the term 'thickness' becomes compromised when you are speaking of a conversion coating which includes some dissolution of the original substrate, transition areas, various different compounds, and a spikey surface.
But if this is zinc electroplating and the density of zinc metal is about 7.1 g/cm3, then you have 610/7.1 = 85.9 cm3 or .0000859 m3 of zinc spread across 1 m2.
So the thickness is .0000859 meters or 85.9 microns or 3.3 thousandths of an inch. That would be too thick for zinc electroplating and sounds more like galvanizing. Typically, and per spec, zinc electroplating would be 5, 8, 13, or 25 microns thick based on whether the service condition was mild, moderate, severe, or very severe.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. 610 g/m2 is the often used equivalent of 85 microns used for hot dip galvanizing.
Can't imagine electroplating that thick!

Geoff Crowley
Crithwood Ltd.
Westfield, Scotland, UK

Calculating the cost of too-thick galvanizing
Q. I am galvanizing steel structures. The zinc micron thickness is higher than the standard coating. For example, I need 85 micron for steel whose weight is 10 ton and I am getting 100 microns for the same weight after checking by Elcometer. The difference of the microns is losing me money, so I need to calculate how much grams I am losing because of the difference of the microns.
John picol- uae
May 7, 2017
A. Hi John. If you multiply the thickness of the coating (100 microns) by the surface area you are coating, you will have the volume of zinc you are using. You can, if you wish, then multiply this by the density of zinc to convert the zinc usage to weight instead of volume.
Your unknown at this point, though, is how much surface area you are coating. Telling us the weight of steel you are coating doesn't help us because if we don't know the thickness of the steel we cannot convert its weight to its area.
As for how to reduce the thickness from 100 microns to 85 microns, we have many threads which address that issue, including:
thread 55032, "How can we minimize galvanize coating thickness",
thread 57597, "Reducing variance in galvanizing coating thickness"
and thread 45551, "Reducing coating thickness by adding nickel salts".
Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. How to estimate cost for chemical consumption per kg production, labour charges per kg ?
Roshan C UgaleSham Enterprises - Nasik, Maharashtra, India
September 12, 2017
Q. I need a GSM of a material with 500 they showed me micron level of 30 on both side totaling 60.
My question is whether we need to take one side of micron level or both sides?
Indway prime energy - Coimbatore, Tamilnadu, India
February 12, 2019
A. Hi Siva. I assume you are saying that you are supposed to offer a coating of 500 grams / meter2 but don't know whether this is per side or total? Most specs mean total weight of zinc on both sides put together (although it's always dangerous to ask a 3rd party to guess what your customer wanted).
It's okay to estimate for your own purposes the grams / meter2 from the thickness, or vice versa, per the examples worked out on this page -- such that, yes, 60 microns average total thickness should be about 430 grams / meter2 -- but I think you are expected to verify the coating weight by stripping and weighing, not by taking a thickness reading at a couple of points and assuming that the thickness will be the same everywhere. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi,
How much zinc coating is needed on 2.45 mm wire to get to 2.50 mm size.
CWI - VANDEBIJLPARK, GAUTENG, SOUTH AFRICA
March 6, 2019
A. That's an easy question. Even easier than 'What is the meaning of life?' If your wire is 2.45 mm in diameter and you want to build it up to 2.50 mm, then you need 0.025 mm of plating, since you plate all around the wire. That's 25 micrometers. And the meaning of life? That's 42. At least according to Douglas Adams.

Tom Rochester
Plating Systems & Technologies, Inc.
Jackson, Michigan, USA

Tom is the author of
"Surface Area Formulas & Tables
- A Resource for Surface Finishers"
|
 Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for instant help or longer-term assistance. |
Q. Sir can we use Elkometer for measurement of galvanize coating.
Sharad Srivastava- rawatbhata rajasthan India
June 11, 2019
A. Elcometer is a brand name of an instrument maker. They make many different instruments.
Your question isn't specific enough.
Which meter do you wish to use?
What do you want to measure?
If you wish to measure the length of the coated item, then a tape measure might do.
If (as I suspect, but you didn't say), you wish to measure the galvanizing thickness, then an instrument is available for this purpose, and ISO 1461, the International standard for galvanizing allows for such an instrument to be used. There are many manufacturers of such instruments, Elcometer is one of many reputable brands.
The standard dictates how many places you should measure, and how to decide where etc.

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

for Engineers, Shops, Specifiers

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon
avail from Amazon
"User's Guide to Hot Dip Galvanizing for Corrosion Protection in Atmospheric Service" by NACE (1997 only rarely avail.)
avail from AbeBooks, or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
Q. An iron rod with 16 mm thickness without any galvanization, then what's the thickness of iron rod after the galvanization?
Rahul Parsodkar- Wardha, Maharashtra, India
August 27, 2019
A. Sorry Rahul, that one is probably unanswerable.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. What is the thickness zinc for 25 mm dia and get 350gsm coating
NILESH KHAMKAR- Pune Maharashtra India
October 13, 2019
A. Hi Nilesh. Several people have said 610GSM is 85 microns, and 430GSM is 60 microns, so 350GSM would be 350/610 x 85 = 48 microns; or 350/430 x 60 = 48 microns average thickness. But again, the math does not guarantee even thickness.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi there,
May I know how many micron is ZF100 Galvanneal coating and the hardness?
Thank you.
steel door manufacturer - Kuala Lumpur, Malaysia
November 21, 2019
A. Hi Mun. ZF100 means the coating weight is 100 grams per square meter (and that usually includes both sides). I think you need to consult a manufacturer's data sheet for the hardness.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. My question is I want to build up 150 microns of zinc coating by hot spraying using zinc wire.
How I will get the exact weight of the zinc to be used.
- Gujarat, India
February 5, 2020
A. Hi Bappu.
Weight = Density X Volume, and
Volume = Surface Area X Thickness
You're making me guess :-)
Do you know the exact surface area you are trying to cover?
Did you know the math, but are actually asking for the density of sprayed zinc to plug into the formula?
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Greetings to all, My name is Rukesh.
I am from Malaysia. I am working in a galvanizing plant which has a production tonnage of almost 1800000 kg to 2000000 kg per month. I have a query regarding the cost comparison between using surface area calculations and also using weight difference.
My steel item has a surface area of: 2.485 m2 (FENCING ITEM)
The thickness of the steel is 5 MM.
The micron we recorded after dipping was 150 Micron which translates to about (150 x 7.2=1080 grams/m2)
Using Weightbridge
The weight of the item before galvanize: 11.5 kg for one piece.
The weight of the item after galvanize: 12.5 kg
Zinc pick-up: 8.7% and almost 1 kg
Using Surface area calculations
Surface area calculations shows pick up of:-
Total grams/m2 zinc deposited: 1080 x 2.485=2683.8 grams deposited on 2.485 m2 of surface area. In Kg, 2.684 kg.
We could observe that for that one piece of weight of zinc pick-up shows:-
For using weighbridge: 1 kg zinc pick up
For using surface area calculations and micron: 2.683 kg.
May I ask why is there a difference in weight by almost 1 kg and also is it more accurate to use the surface area calculations compared to weight difference of incoming and outgoing?
The comparison is made with the same item and of one piece sample size.
Would really appreciate if I could get a feedback regarding this matter as the difference in weight is quite significant.
Thanks.
Best regards,
Malaysian Mega Galvanizer SDN. BHD - Petaling Jaya
February 18, 2020
A. Dear friend,
After pickling the weight will be less. For example if your material's weight was 11.5 kg before galvanizing, but after pickling the weight will be 10.5 kg (supposed). Also the density of Zinc is not exactly 7.2, So here are some errors. That's why you observed the deviation.
- Mymensingh, Bangladesh
September 27, 2023
A. There are always arguments about zinc consumption, actual zinc on an item, and about where the zinc goes to.
Your approach coming from thickness measurement of 150µ, sounds like a single thickness measurement? On an item of your stated 2.485m2 you would likely find thickness measurements ranging from about 80 to 2000µ. It will vary across the dimensions, with the longest immersion area (first in, last out) parts thickest coating, and opposite parts (as hung) will be thinner. At the last drip point there could be 2mm thickness. You'd need to have a carefully devised plan of many measurements to get an average thickness.
Taking weight into the process and weight out also has its problems. The pretreatment process will remove some weight of the original unprocessed steel item. Then the zinc adds weight. So there's 3 stages of weighing could be considered.
Your stated weight difference of 8.7% is excessive. If it's correct, then your process needs urgent attention. A 5mm thick item should gain about 5% weight.
How did you weigh this piece. You mentioned a weighbridge. Typically these are made for weighing a vehicle and its load, perhaps 40 tonnes. Even a small one for 10 tonnes has an accuracy of +/- several kg (say 2 kg in 10,000, or 0.02%). So to measure 2 kg is almost impossible.
So your question was "why is there a difference between two systems of measurement?". My answer is that both methods have such a level of inaccuracy that both answers are a bit meaningless, and a comparison between inaccuracy and inaccuracy is also meaningless. Sorry, but repeat the experiment with more accuracy and design of the errors involved.

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

September 28, 2023
Q. Sir can you tell me the formula for converting microns to gsm in metals?
Katakam saathwik- Warangal, telengana, India
February 20, 2020
A. Hi Katakam. As you can see from the above question from Rukesh, it can be a bit difficult in practice, but in theory it's very easy ...
Weight = Volume X Density, and
Volume = Surface Area X Thickness, so
Weight = Surface Area X Thickness X Density.
Because there are 10,000 cm2 per m2 of Surface Area, and 1/10,000 of a cm in a micron, the numbers work out nicely, with 1 micron thickness over a square meter being equal to 1 cm3 of metal. So the gsm per micron thickness is, happily, simply the density of the metal per cm3.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Sir, I have sample wire of 1.6 mm dia. Before zinc coating its weight is 3.19 gm, and after 3.27 gm. So I want to calculate zinc coating weight in gm/m2. Please explain with proper formula and brief calculation.
Mahendra ChoudharyIndia - Jaipur, Rajasthan India
March 15, 2020
A. Hi Mahendra. If you don't know the substrate material, this problem is unsolvable because, without the length of the wire we have too many unknowns so we can't calculate anything. But if this is a galvanizing situation so we can assume that the substrate is steel, then --
1. Since you know the substrate material, you can estimate its density.
2. Now knowing its weight and density, you can solve for the volume of wire you have.
3. Now knowing the volume and the diameter, you can solve for the length.
4. Now knowing the length and diameter, you can solve for the surface area.
5. Finally, now knowing the added weight of the zinc coating and the surface area of the wire, you can solve for the gm/m2. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. My steel plate of 920 x 375 x 1.4 mm
Weight - 4000 gm
Coating - 67.5 micron
How to calculate GSM of that plate
- Ahmednagar, India
May 11, 2020
A. Hi Swapnil. If you ask a question you don't really understand, the answer isn't of much value; are you sure you understand the question? (The reason I ask is because you gave five pieces of info and four of them are extraneous: neither the dimensions of the plate nor its weight matter).
If a coating is 67.5 millionths of a meter thick, the volume of the coating on one side of a square meter of surface is 67.5 millionths of a cubic meter, and zinc weighs about 7140 kg per cubic meter, so the weight of the coating on that square meter is 0.482 kg or 482 grams per side. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. We are using continue galvanizing furnace for wire rod zinc plating of dia (1mm to 5mm). I want to know how to calculate the DV of galvanizing furnace, zinc thickness for formula for different wire dia., and how to calculate speed, and dip length.
Dinesh Kumarengineering student - Ludhiana, India
May 31, 2020
A. Hi Dinesh. I'm not a galvanizer and can't answer your questions. Sorry! But as site curator I think you'll improve the chances of readers spending their time by realizing that people would like to learn something from you ... and they want to help an actual individual ... and they seek the satisfaction of helping to solve an actual problem ... and most of them don't relish just looking stuff up in books and quoting it.
So if you don't get quick responses, please humor me and try to add to your question in one or more of those ways. Thanks!
Oh! And one more thing people sometimes like to do: if they feel that someone is "lecturing" someone else, they like to prove them wrong. So maybe my posting will help after all :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi.
I outsource steel parts for galvanising and would like to verify how I'm being charged for zinc consumption.
E.g., For a 2 mm thickness plate that weighs 15.7 kg per square metre, at 100 microns - what would the weight of zinc used per kg of this plate?
- Hyderabad India
September 24, 2020
A. Hi Anu. I wish I could find a plumber who would charge me by how many washers he replaces :-)
I doubt that you are actually charged by the volume of zinc consumed because labor, handling, maintenance, and overhead are actually involved.
But the density of zinc is 7.133 g/cm^3 or 7133 kg/m3. So if the coating is 100 microns thick on each side, the volume of zinc deposited on a square meter of sheet would be more or less: 2 sides x (100 x 10^-6 m) thickness x 1 m^2 area = 2 x 10^-4 m^3.
And the weight would be roughly (2 x 10^-4 m^3) x 7133 kg/m^3 or 1.427 kg.
I've been pleased to work examples on this page just to make sure there have been no misunderstandings about concepts. Ask away about any concepts you're not following but, sorry, I'm not doing additional arithmetic problems :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. As a galvanizer, I can confirm what Ted has said. Most galvanizers don't charge on zinc consumed. They might charge extra for something that consumes a lot of zinc, but the variability in zinc consumption from one job to another isn't enough to compensate for the variability in factory space it occupies.
You will typically get charged a lot for something that...
* is lightweight
* Has high surface to weight ratio
* Has a high handling element (lots of small items)
* is hollow (and so gets galvanized inside as well as outside)
* Takes a long time to dip (holes too small)
* Is a trivially small order (where the administration costs more than the galvanizing)

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

Q. I need to verify Aluzinc (Galvalume) coating of a sheet. I searched on internet and found that density of Aluzinc is less than conventional zinc coating. The Aluzinc contents seems to be 55% Al, 42% Zn & 1.5% Si. I checked coating thickness of sheet and found that coating thickness is nearly 35 microns (each side). The base metal thickness is 1.55 mm. Using weight method with formula (m1-m2)/A*1E6 where mass in gram & Area in mm2.
m1-27.06 g & m2-26.60 g
Area of coated sheet - 40.52x54.70 mm2
My question is how do I correlate coating thickness (in microns) with coating mass (in GSM)?
What if coating is only at one side? What will be the coating mass?
- Vadodara Gujarat
October 26, 2020
A. Sounds like fairly simple arithmetical question to me. You have stated all the parameters, and Good will give you those you don't have, so you can calculate this easily yourself.
There's something wrong if you need this spelled out.

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Dear Sir,
considering that dry ash is 1% and dross 0.6% of galvanised material weight what should be the ratio between zinc pickup and gross zinc consumption?
How much should be the weight increase in galvanised steel in case of 6mm and 3mm thick steel considering different micron coatings?
- kolkata, west bengal, India
October 23, 2021
A. Hi Vikash. I'm not really understanding your first question. If you are saying you are losing only 1.6%, it would seem that zinc pickup would have to account for the other 98.4% of consumption ... what am I missing?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Numbers to aim for with the thickness of steel you quote...
Dross: 0.3 to 0.9% of the weight of steel galvanized (white weight)
Ash: 0.2 to 0.5% of the weight of steel galvanized (white weight)
Total zinc pickup should be about 4-5%
There are usually easily identifiable reasons for having greater losses than this.

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

Q. How can I calculate pipes zinc coating? Example: 48.30 mm OD x thickness 3.00 mm x length 6.00 mtr (coating 40µ)
Please tell me formula to calculate pipe coating.
QC - united arab emirates
January 13, 2022
A. Hi Yassar. If you know the total surface area of something and the thickness of the coating, you simply multiply them together to get the volume of the coating. And then you simply multiply by the density of the coating to get its weight. But the tricky part is figuring out the surface area. For a hollow cylinder, the formula is:
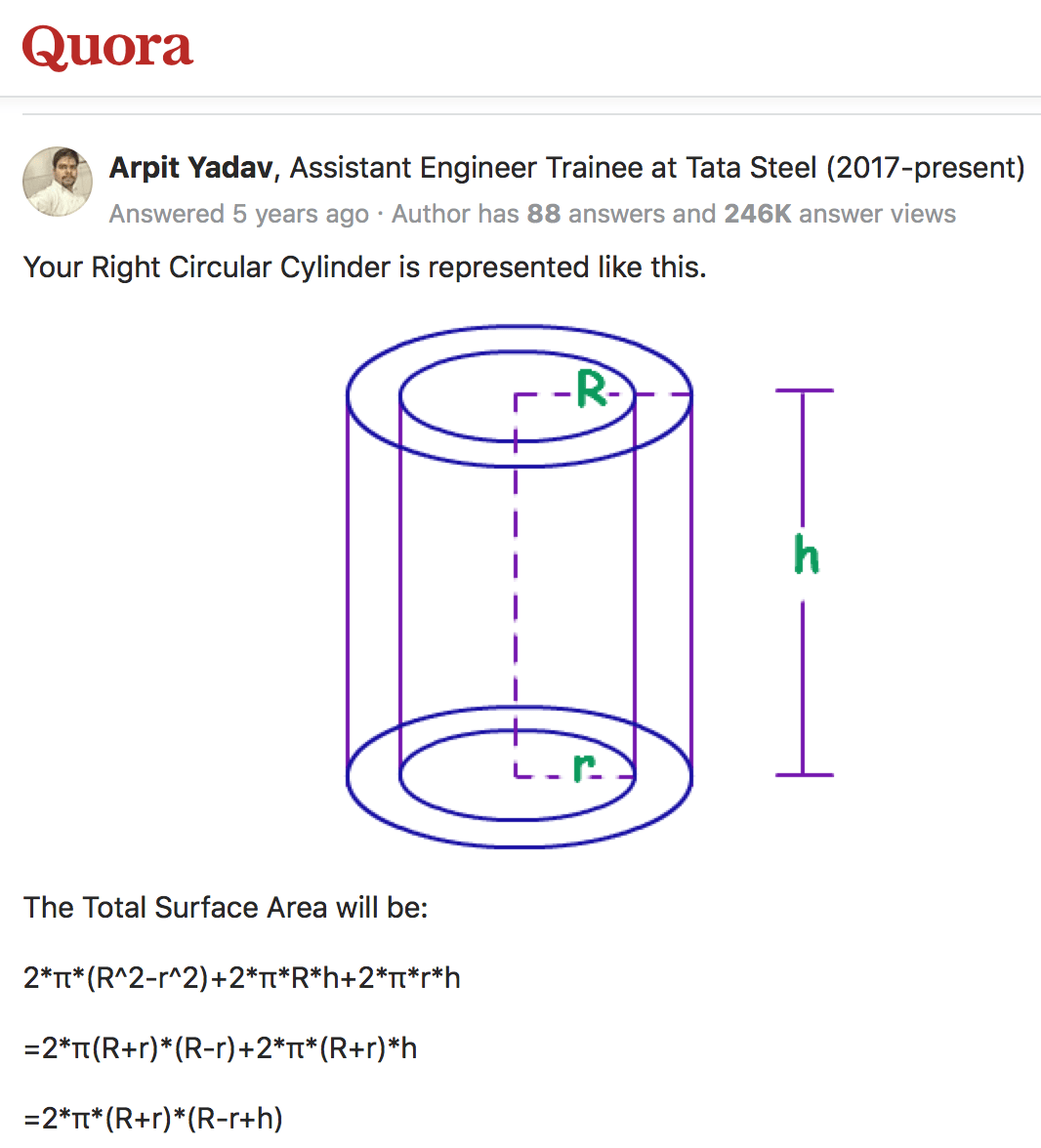
You might be interested in Tom Rochester's book, "Surface Area Formulas and Tables", which explains a lot about estimating surface areas. For example, when the wall of the tubing is thin enough, you can ignore the tiny area of the tube ends, and the difference between the inside and outside diameter and estimate the surface area as approximately 2 * π * OD * length. In your case the simplified formula is 99.38% of the actual.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Not sure what you are asking, as you have stated the thickness of the galvanizing to be 40 µ
If you want to calculate the weight of zinc on a single pipe, then calculate the volume of zinc and multiply by its density (SG. Zinc is about 7.1 as a solid, 6.9 as a liquid)

Geoff Crowley, galvanizing consultant
Crithwood Ltd.
Bathgate, Scotland, UK

Q. How do we calculate zinc used while galvanising steel coils?
E.g. - if base metal thickness is 0.22 mm x width 850 mm x 681000 mm and zinc coating including both sides is 40 gsm/sq meter?
And how does the zinc thickness and consumption change with change in thickness? I zinc coating gets changed to 25 gsm/sq meter and 80 gsm/sq meter?
self employed - Mumbai, Maharashtra, India
April 11, 2022
A. Hi Manish. You used the nonsense term "gsm/sq meter" three times in your short question, so I don't think it's a typo -- but rather that you may be attempting to ask for the answer without quite understanding the question :-)
That approach, mathematically solving for the answer without truly understanding the question, is reserved to quantum physicists, not we mere mortals :-)
"GSM" is an abbreviation/acronym for "grams per square meter", so "gsm/sq meter" would mean "grams per square meter per square meter" and makes no sense. I think that might be why the simple arithmetic of your question is puzzling you. Use either "grams per square meter" or its acronym "GSM".
You know the area of your coil in square meters: The area of the top surface is 850 mm x 681000 mm; the area of the bottom surface is the same; the area of the left edge is 0.22 mm x 681000 mm; the area of the right edge is the same. But 0.22 is SO much smaller than 850 that the area of the edges can be safely ignored. So figuring just the top and bottom, the area is 2 x 850 mm x 681000 mm, i.e., 2 x 0.850 m x 681 m, or 1158 square meters.
Multiply that number of square meters of area times your grams per square meter to arrive at your grams.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread

