
-----
Why must Copper Bus Bar be Tin Plated?
Q. Is it necessary to put bimetallic strip in joints of a tinned copper busbar & aluminium busbar? Please explain.
Gunjan Singh- Maharashtra, India
July 31, 2013
A. Hi Gunjan. Sorry but I don't understand the question. To my knowledge unplated aluminum busbar is not really satisfactory, although I have seen it used with No-Ox-Id compound for joints permanently bolted to copper. I've never personally seen "bimetallic strips" in any joint, although this 3rd asking of the same question might indicate that maybe I'm just ignorant about a commonplace approach :-)
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. MY QUESTION IS WHY DO WE HAVE TO PUT HOT COPPER BUS BARS IN WATER AFTER TINTING ^TINNING?
ARE THERE SOME DANGER/HAZARDS IF YOU DON'T DIP THEM IN WATER, AND WHAT TYPE OF HAZARDS?
- CAPE TOWN, SOUTH AFRICA
August 14, 2013
A. Hi Simphiwe. Sorry but I don't quite understand. Please explain what you are doing. Thanks.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
August 15, 2013
A. If you're talking about rinsing, it's important to remove the plating chemicals. Not only is it important to ensure quality, it is also for safety and many other reasons. It's not a huge danger but rinsing parts after plating helps keep the workplace clean and provides safety for the people using the items amongst multiple other reasons.

Blake Kneedler
Feather Hollow Eng. - Stockton, California
August 15, 2013
Q. Dear Sir
As we are aware the resistance of tin is more than copper, so will tinning of copper on the contacts affect the current flow?
- Thrissur, Kerala, India
September 6, 2013
A. Hi Shabeeb. Tin plating will improve the current flow because the tin does not oxidize as badly as the unplated copper, so it makes a better contact surface.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
September 6, 2013
Q. Dear sir,
Is zinc coating for copper busbar as good as tin plating?
What is better between coating zinc or tin?
Regards,
- Pune, India
September 27, 2013
A. Hi Asif. Zinc plating will form gummy, resistive, corrosion products. Zinc is certainly not an acceptable plating for bus bars. Sorry.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
September 27, 2013
Q. Sir,
What is the effect of tin plating on the copper. Remember the skin effect current is passing through the outer surface of conductor. We have tin plating on the copper, now copper is the conductor. Tin has different resistance and copper has different resistance. Now my question is: tin will affect the total resistance of the copper bar because of tin plated on the copper? In this case copper resistance is affected or not.
Please give detailed answer.
Regards,
Jack
Student - Duabi, UAE
February 5, 2014
A. Hello Jack. Very high frequency currents do tend to travel on the "skin" of a conductor rather than through it's core. But the study of that phenomenon is an electrical engineering discipline which is a subject of greater depth than can be handled here, off-topic for this metal finishing site, and probably not what you were asking anyway :-)
I only mention it to not mislead people who may have found this page because of the search term "skin effect", which we are using in a different way than they might.
We are talking here about bus bars, and they are used for DC current or low frequency AC current, where the current flows through the bar, not on its skin. The resistance of a bus bar, and thus its current carrying capacity, are related to its resistivity as follows: Resistance = resistivity X length ÷ cross-sectional Area.
Since 99.9%+ percent of the cross sectional area of the bus bar is copper, the bus bar's current carrying capacity is not measurably increased or reduced by plating the bar with another material.
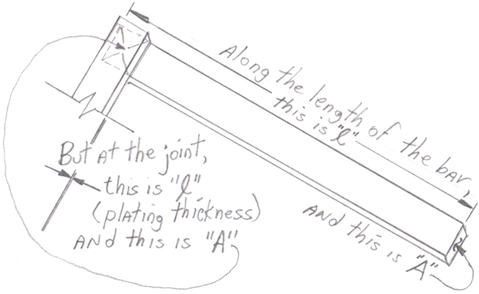
However, getting the current into and out of the bar requires that the contact faces be conductive rather than resistive, and silver or tin plating will do a better job than copper for the reasons already discussed on this page, offering a less oxidized skin. Remember that when you are calculating the resistance across the joint rather than along the length of the bar, per "Resistance = resistivity X length ÷ cross-sectional Area", now "A" is the cross sectional area of the joint and "length" is the thickness of the plating. So this piece of the Resistance is extremely minor because "length", the thickness of the plating, is next to nothing. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 2014
Q. Ted,
If installing a tin or silver plated copper bus bar as a "bay" frame ground for terminating EQP grounds within the bay, is there a need to utilize No-Ox-Id between the EQP 6 AWG ground lead and the mounting surface position of the buss bar? If not, is it because the plating of the bus bar coupled with the fact that Telco sites are controlled environments negate the need to utilize No-Ox-Id?
Thanks
- Trabuco Canyon California US
February 12, 2014
A. Hi Antoino. I have a good deal of experience in electroplating shop environments, and a small amount as an amateur electrician. But I am not qualified to comment on codes or best practices in your field. All I can say is that, if you are not in a cleanroom environment (which might discourage use of compounds), and there is no other reason to specifically discourage joint compounds, it would seem that electrical joint compound is always a good idea.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 2014
Q. Hello,
If you are using tin plated copper bus, is there any advantage to silver plating the connection point?
Thanks.
- Regina, SK, Canada
May 20, 2014
A. Hi Paul. I doubt it, and I've never personally heard of it.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 2014
Must we de-rate copper bus bar if we tin plate it?
Q. Hi,
We have 658HP VFD drive(415V/3ph/50Hz) & installed in factory where the surrounding is corrosive due to which the copper bus bar & DC bus plates are getting blacked. We are planning to do tinning to all copper bus bars & DC bus plates. So want to know whether to consider any de-rating of copper when tinning or other way is copper bus bar current capacity de-rate due to tinning?
- Mumbai, India
August 19, 2014
A. Hi Sayed. I would not anticipate any need for de-rating based on my understanding of the situation. However, there still may be codes or "best practices" that I would not be aware of since large horsepower variable frequency drive installations are not my field.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
September 2014
Q. I would like to know, is there any requirement for the copper bars in a busbars to be plated with Tin along the entire length as the bars are insulated with Epoxy, which is electrostatically deposited on to bare bars.
Bibin Joy- Dubai, UAE
September 17, 2014
A. Hi Bibin. I can't answer that question and I doubt that anyone can because you seem to be asking about job requirements or specification requirements rather than a technology question. But certainly I would expect an epoxy coating to deliver even better corrosion resistance than tin plating. Best of luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
September 2014
A. Tin will actually slightly increase the conductivity of the buss bar because you are adding metal.
Conversely, powder coat is an insulator and will not let the bus bar dissipate the heat to the atmosphere as well as the temp goes up, so does the resistance which will generate more heat. So, your operation will probably be less efficient.
- Navarre, Florida
September 27, 2014
Conductivity of bus bar is too low
Q. Please, I need help regarding bus bar manufacture.
We are producing copper bus bar (OFE COPPER BUS BAR) but sometimes it comes with low electrical conductivity. WHAT CAN MAKE THIS CONDUCTIVITY LOW?
- RIYADH,ASIA,SAUDI ARABIA
September 28, 2014
A. Hi Ahmed. Our focus is finishing and I know little about copper smelting. But the conductivity of copper declines drastically with even a very small amount of contamination because any impurity interrupts the travel of the free electrons. Are you absolutely sure the copper is electrolytically pure?
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
September 2014
Can we use galvanized hardware to join copper bus bars
Q. Can we use galvanized steel to assemble the panel copper bus bars?
khaled abo talib- riyadh, kingdom of saudia arabia
November 10, 2014
Q. An electroplating plant has bus-bars which carry up to 1000 A at 6 V dc. The surroundings are very wet with water and electrolyte. Should the bus bars be insulated and if so, why?
nurul syazmeen- - melaka, malaysia
April 3, 2015
A. Hi Nurul. No, the bus bars should not be insulated. But the bus bars are not the problem; the problem is that the surroundings are very wet, and you should examine carefully any assumptions about what conditions are unavoidable. Please see my article "Plating Shops for the New Millennium". Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 2015
Q. Please, regarding manufacturing of tin plated copper bus bar: anyone can guide me to specification for electroplating of tin on copper?
Which specification can I follow? What is the minimum AND maximum tin thickness ON COPPER BUS BAR?
- riyadh-SAUDI ARABIA
April 26, 2015
Q. Sir,
I want your help.
At present we are facing problem of High temperature in our 6.6/11 KV Bus Bar Joint, CT Connection joints temperature is up to 120 °C
I want to know what best solution to avoid this is.
Thanks
- Jamnagar, India
July 31, 2015
A. Hi Shekhar. We have posted your inquiry and hopefully someone will help. My experience is in metal finishing, not switchgear design, although it sounds like the conductors are probably too small for the current they are carrying.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
August 2015
Q. I am working on a lighting project that uses a 10 AWG equivalent round copper busbar for low voltage track lighting. The voltage is 12V AC or DC, and the maximum current through the system is 20 Amps.
The sections of the track are joined with spring-loaded blades that slide against the copper busbar, much like the typical track lighting interconnects you would see at Home Depot.
My questions are:
1. Do you think I need to plate the copper busbar to ensure connection reliability, or would bare copper by acceptable due to the low voltage and relatively low current?
2. If plating is necessary, would nickel plating perform reliably compared to tin plating? Our contract manufacturer already nickel plates many parts for aesthetic purposes, but they don't do tin plating. So nickel plating would be familiar to them, but I don't know if nickel plating has any significant disadvantages over tin plating.
Thanks!
- Chicago, Illinois, USA
August 21, 2015
A. Hi Chris. Again, I don't portray myself as an expert in such subjects by any means ... but I don't see any need to plate the copper. Make sure, of course, that you are using electrolytic grade copper as even a small amount of impurities has a devastating effect on electrical conductivity.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
August 2015
Q. Whether tinned copper bus bars are required in a medium of SF6 gas. Whether the current carrying capacity gets de-rated in SF6 gas medium?
Manas Bhattacharjee- New Delhi, India
December 1, 2015

A. Hi Manas. I know nothing of SF6 switchgear, but certainly any joints would seem to benefit from tin plating irrespective of what medium the joint is in, because you will not be manufacturing the copper and assembling the joint within an SF6 environment, but out in the open.
Please try to detail your actual situation rather than casting the proposition in the abstract with sentence fragments :-)
There would seem to be no air cooling within a pressurized SF6 environment, so presumably you must de-rate accordingly. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
December 2015
Ed. note Aug 2018: The illustrated paper is no longer available from the original source, but can be found at archive.org by inserting its original URL, viz., http://www.tdeurope.eu/data/file/Environment-Sustainabilty-Approach-Capiel-HV-Part-D-Switchgear-and-SF6-Gas.pdf
December 22, 2015
Q. Dear All
I had a question, what grade of copper is being used and which will be the best?
Grade:
- ETP Grade - IS 613, BS 2871C-101 or
- DHP Grade - IS 2501-DHP, BS 2871C-106.
What is the main difference between both grades.
Which will be used in Bus ducts system and Panel boards ?
Please provide me with details.
Regards
bus bar manufacturer - Riyad, Saudi Arabia
Discoloration of tin plated bus before it's even used
Q. Why black colour marks are formed on the tin coated busbars of the new panel board which not yet charged?
Raviraj Channalliautomation - Bangalore, Karnataka, India
January 18, 2016
January 20, 2016
Q. I am struck in this situation, hope someone will extend a helping hand
A copper cable to be connected(either crimped/ soldered/ pressure/screw fitted) to stainless steel bus bar and the bus bar is to be connected to the power supply of 12 V DC.


Now I have the following queries:
1. Can a connection be possible from copper wire to Stainless steel bus bar ? If yes, what are the factors need to be considered ? Can copper wire be connected to copper coated stainless steel bus bar ?
2. What type of connection between copper wire and steel is feasible (crimping, clamping, screw etc).
3. In my case, coating of stainless steel with any metal will make the connection feasible? if possible also the electric transfer efficiency of the connection in various cases?
Thanks in advance...
- Coimbatore , Tamil Nadu, India
A. Good day Abhilash.
I think you should consider the current carrying capability of stainless vs. copper buss bar. The "electric transfer efficiency" of stainless is much lower than that of copper.
Connections can be clamped or bolted with the use of an oxide grease. They can also be clamped or bolted and soldered.
Hope this was helpful.
Regards,
Aerotek Mfg. Ltd. - Whitby, Ontario, Canada
January 21, 2016
Q. Coating the stainless steel bus bar with copper can solve the issue and establish an efficient power connection in my case?
Or plating of the bus bar with any metal will be helpful in my case?
- Coimbatore, Tamil Nadu, India
January 22, 2016
A. Hi Abhilesh. I think that Eric was suggesting that stainless steel is not a very good bus bar substrate material -- it's current carrying capacity is only about 10% of the current carrying capacity of copper bus bar. But if you will be using stainless steel anyway, yes, it must be plated.
Copper plating should be sufficient for a 12 V power supply situation if the connection is tightly bolted, and a compound is used. But copper plating the bus bar, and then silver plating all of the contact surfaces would be better. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
January 2016
Q. Hi,
Two questions,
1. Is is advisable to place a bimetallic washer in between a tinned copper and tinned aluminum termination of an electrical joint?
2. We always find some white powder over the tinned copper lugs. It is actually a Cu-Al joint? What and why is that white powder present there?
Please answer as I didn't find any answers so far.
- Chennai, tamil nadu, India
January 24, 2016
Q. Hello Sir,
We are using the copper bus bars with Tin plating (8 to 10 microns thickness). Some of the bus bars are Silver plated. These silver plated bus bars are oxidized (light black shades). So which type of solvent can be used for cleaning the black shades? Can you please suggest the solvent?
- Hyderabad, Telangana, India.
February 6, 2016
A. Hi Murthy. You're the second person to ask that this week, and my answer until I hear otherwise is that I don't know why everyday silver polish (used for flatware) could not do this job. If you google "MSDS silver polish" you will see lists of important ingredients. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 2016
Q, A, or Comment on THIS thread -or- Start a NEW Thread

