
-----
Chemicals for nickel plating hard to find
Q. I am looking to purchase a small amount of nickel salt to try to do some nickel plating. In addition to the Dibuthydithiocarbamate I will also need a small quantity of sal ammonia. Can someone tell me where to purchase a small amount? Also why is it so hard to find?
Paul Primmer- Columbus, Ohio, USA
2004
A. Hi, Paul. Please google with the phrase "lab supplies" to find companies like Fischer Scientific who specialize in small quantity specialty chemicals like these rather than those dealing in large scale bulk chemicals. Also try googling for "hobby plating chemicals". Good luck!
Yes, product stewardship of chemicals turned the once-simple process of buying chemicals into a hassle. Suppliers worry about what might go wrong and often feel it is a losing proposition to sell small quantities today, so they don't. But you may be able to find suppliers in your local area under 'Plating Supplies' though.
Virtually all nickel plating is done with proprietary processes these days, rather than home-brew mixtures. It's not clear to me whether you've a particular reason to want to formulate your own nickel plating solution or if if packaged nickel plating processes will satisfy you. Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2004
Q. Hello,
A. I see you already have a protocol as you're mentioning a compound (Dibuthydithiocarbamate). Can you tell me what your protocol is as the ones I have are old fashioned ones and you seem to have a more fancy one that I may use!
Thanks,
 Ted Mooney, P.E. I've read many of your comments and they don't help. Why don't you start helping out (isn't it the purpose of this forum?) instead of bringing people down? Or then, stop answering questions; it's a waste of time for us, maybe not for you as it may be the way you fulfill your inferiority/superiority complex...
Ted Mooney, P.E. I've read many of your comments and they don't help. Why don't you start helping out (isn't it the purpose of this forum?) instead of bringing people down? Or then, stop answering questions; it's a waste of time for us, maybe not for you as it may be the way you fulfill your inferiority/superiority complex...
- san diego, California
2004
![]() Hi Michael. You're certainly free to hold a low opinion of me, but ad-hominems derail conversations not advance them.
Hi Michael. You're certainly free to hold a low opinion of me, but ad-hominems derail conversations not advance them.
Paul asked why chemicals are hard to buy in small quantities so I spoke the hard truth from inside knowledge: most companies don't want to sell chemicals to hobbyists and very small businesses. He asked where to get them anyway; I did my best to answer. People naturally assume they must make up plating solutions from raw chemicals because that's what they see in science class; I told him that wasn't the case in case he didn't know yet.
Have a good day!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2004
March 13, 2020
Q. I have a serious question and have never been able to get an answer from any source yet. I make vintage replica items for musicians. I am making nickel silver covers and plating them myself using the [brand name deleted by editor] products. I am using an aging method, mostly using ferric chloride
⇦ on
eBay or
Amazon [affil link] . The problem with [brand name deleted by editor] nickel plating materials, probably. Their BRIGHTENER, in particular, is nothing like the nickel plating done on parts in the 1950's. These old parts do not retain the bright finish and tarnish quite easily.
I know that some companies are still plating this way, because I've been able to age some nickel plated parts very easily, but am not able to do it to my plating.
I am assuming they are using maybe some CHROME? In their brightener that simply will not let me use acid darkeners or ferric chloride to age my plating. I have seen on some supplier sites that they offer brighteners that are not super shiny and offer them in various degrees. However, none of them will answer my emails and my attempts to buy in very small quantity so I can do a plating that I can easily AGE. Any information would be just wonderful, as I am battling this problem going on 15 years or more, and am having to resort to extreme measures to get the kind of vintage "looks" I am trying to copy. I am including a photo of a vintage article and how the nickel plating ages on these articles.

Thanks for your time, hope to hear back with sources or knowledge,
Dave StephensOwner small business, looking to replicate vintage items - BATTLEGROUND, Washington, USA
9th Edition, Vol. 5
"Surface Cleaning, Finishing & Coating"

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Dave. Sorry, we had to delete the vendor's name from your posting (why?). But I doubt that their nickel plating solution concurrently deposits chrome because that is virtually impossible to do.
Since you are obviously putting a lot of effort into this, you might see if a library within driving distance has the ASM Metals Handbook Vol. 5 because it has a great, practical, chapter on nickel plating including naming some old-fashioned addition agents. You might try plating with a Watt's Nickel dull, or semi-bright, or even brightener-free nickel bath and manually buffing to smoothness if necessary. You can search the site for lots of info on Watt's nickel formulation, operation, and troubleshooting. You might also find thread 28011 and 38403 quite interesting. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Thanks for the reply. It was pretty confusing, but you sent me on the right trail and I think I found what I want to do. A guy did a little Youtube simple Watts bath demo on a penny and it came out nice and bright looking with 3 simple ingredients. From there I tracked down more information on the semi bright and bright mixes and I think Watts may be exactly what I'm looking for. I did once plate without any brightener but it came out kind of matte looking and polished up fine, but it didn't look quite right and didn't age like the vintage articles I have. So, I'm going to order the ingredients, mix it all up and pray it nails what I want to do. The information on brighteners is just way too confusing for my "pay grade," so won't even attempt that. Wish me luck,
Thanks for the reply. It was pretty confusing, but you sent me on the right trail and I think I found what I want to do. A guy did a little Youtube simple Watts bath demo on a penny and it came out nice and bright looking with 3 simple ingredients. From there I tracked down more information on the semi bright and bright mixes and I think Watts may be exactly what I'm looking for. I did once plate without any brightener but it came out kind of matte looking and polished up fine, but it didn't look quite right and didn't age like the vintage articles I have. So, I'm going to order the ingredients, mix it all up and pray it nails what I want to do. The information on brighteners is just way too confusing for my "pay grade," so won't even attempt that. Wish me luck,
Dave
- BATTLEGROUND, Washington, USA
March 13, 2020
Saccharin brightener in Watts Bright Bath?
Q. I ordered supplies to do a basic Watts nickel plating bath as per your helpful recommendations; I greatly appreciate your time.
I have read that saccharin is a good brightener to use, will it be anymore brighter than the higher nickel chloride Watts bath? Can I use it in the higher chloride bath recipe?
I seem to have read that SODIUM saccharin works better? Bear in mind I am amateur plater and have been using hobbyist materials from a well know hobby plating vendor to plate my commercial low volume products. So, this will be my first use of mixing my own chemicals to achieve a vintage method of bright plating that I can easily age.
So, I cannot find out how much sodium saccharin to use per gallon? Need real world advice here, and can't find an answer in the Volume 5 ASM book you recommended. Again, thanks for your time in sharing your professional time.
Dave Stephens
- BATTLEGROUND, Washington state, USA
March 15, 2020
A. Hi again. The ASM Handbook suggests 0.07 to 0.5 oz./gal of sodium saccharin, and notes that it's not consumed by the plating process. So probably start on the low end ... but personally I'd try with none first.
An amateur plater with no instrumentation or lab experience formulating his own nickel plating solution is going to find the going very difficult; and if that wasn't tough enough, you then want to artificially age the plating with ferric chloride ⇦ on eBay or Amazon [affil link] :-)
But this site has several threads about early nickel plating with good links to follow, like
https://nickelinstitute.org/media/2323/nph_141015.pdf
and
https://www.nmfrc.org/subs/history/hist.php
Real-world advice on a hobby that not many are engaging in, and which uses practices which commercial platers don't, is unfortunately hard to come by :-(
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 2020
March 15, 2020
Q. I've never had a problem with being an OCD research driven guy ;-)
I've been plating for 15 years, using hobby resources, admittedly, but I am bound and driven to master this Watt's bath method for what I'm doing, and I think I can make it work. I am 70 years old and have a niche product, that involves small scale machining, plating and other craftsman/artist processes. I do have an adjustable rectifier DC plater transformer, and large nickel plates, so am not really starting from the beginning. I have those same 15 years of experience using ferric chloride to simulate 60 years of human acid sweat etching on vintage nickel plated surfaces. It works incredibly well, but on modern nickel plated surfaces its quite difficult, and involves abrasive sanding to break the hard glaze of the plating so the FC can more easily etch it successfully. This involves multiple abrasion and FC etching, and other techniques I've developed to eat away plating down to base nickel silver. I think Watts plating will simplify my aging techniques because I don't think its a surface that would survive the long process of destroying the really hard nickel plating designed to not tarnish EVER ;-)
I will do as you suggest and do the basic bright Watts nickel plating, and will have to experiment with voltage and amperage, but it's probably the same as I am doing now. I will attach a photo for your amusement of a super age/destroyed plating to show you the most extreme version of what I'm doing.

I do really appreciate your help, and you're right, I don't even know anyone in my business who is plating their own covers, nor coming close to my aging looks, nor would even put in the insane hours I do to invent these techniques that no one can figure out what I'm doing, LOL. Wish me luck ... Dave
Dave Stephens [returning]- BATTLEGROUND, Washington, USA
![]() Thanks, and good luck. For sense of scale are they electric guitar pickups with the distance between screws the distance between strings?
Thanks, and good luck. For sense of scale are they electric guitar pickups with the distance between screws the distance between strings?
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 2020
April 4, 2020
Q. OK, I spent a fortune on the Watt's chemicals. I took the maximum weights from the Wikipedia chart for "bright" chemical mix, and used 80% of the max, in a gallon and a half. It's all mixed up and ready to go. But the pH reading isn't up to what they show it should be. They show 4.7-5.1 pH. I'm getting 3.42 pH. Please see my chart and what I mixed up.
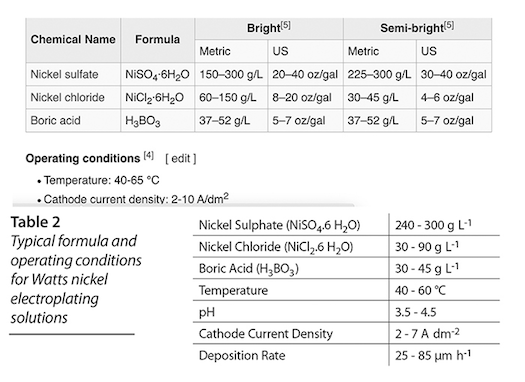
Should I mix in the 20% of the chemicals that I left out? I was a little worried about having too saturated a mix, which is why I didn't use the maximum weights allowable.
I"m scared to death of ruining the bath, its more than $100 in that pail. Plus I don't want to ruin numerous covers.
Hope you can give me some pointers here, this is costing a lot of money and have very high hopes this will be what I need.
Thanks,
- BATTLEGROUND, Washington, USA
A. Hello again, Dave. We have dozens of threads on this site about Watts Nickel plating, so Wikipedia may muddy the water rather than clarify it ... but I don't see where they suggest a pH of 4.7 to 5.1 ! The chart you posted says 3.5-4.5.
Don't add more concentration, and don't touch the pH for now, it's only slightly below 3.5 and will rise in operation if you operate correctly. You should be able to successfully plate from what you've made up.
But your general approach of making up the whole gallon and a half for experiments is not ideal. Although you probably don't have a Hull Cell ⇦ huh? , and I'm not advocating that you spend still more money, what people generally do is plate test panels in a 267-ml (about 1 cup) cell and scale up from there. Please spend some time searching the site, and don't miss Jeffrey Holmes posting on thread 55561, or the two threads I suggested earlier. Best of luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 2020
April 6, 2020
The pH was cut off the image is all. I found another source that's not so crazy as Wikipedia anyway.
So, am completely discouraged tonite. The "bright" Watts bath didn't do anything but plate pure dull matte nickel. Not what I was hoping for. I wanted the covers to come out of the bath BRIGHT. No polishing needed. I'm sending a photo of what came out. The dark specks are not related to the plating so ignore those.

I have 1950's covers that are bright nickel and have stayed bright all these years. If I dab nickel darkener acid on them they instantly go black. If exposed to ferric they instantly lose shine and get a little dark. The hobby brightener I use so incredibly hardens the nickel into a shine that cannot be "aged" without alot of scrubbing and torturous work to even come close to making them look aged. This is why I am trying to find out how to duplicate the plating on these 50's parts. I don't think they just plated matte nickel then polished it, because the inside of the covers are bright nickel. So maybe what I am hoping isn't possible?
I was hoping to find a company that sells nickel brighteners that aren't so bullet proof that they never tarnish, but the one company I wrote to wouldn't reply and probably weren't interested in selling me a small quantity.
If you have any other suggestions, believe me I appreciate your level of expertise, and believe me I have SCOURED your website and not found what was looking for. Also cannot find how the current density formula works. 2-10 A/Dm2 Does that mean Amperes divided by squared meters? I used 2 Amperes to plate this piece, according to the easy to understand hobby plating settings.
Dave
- BATTLEGROUND, Washington, USA
A. Hi Dave. None of us know what we don't know. I don't even understand what look you are going for. I don't think we're even agreeing on the meaning of the word "bright". Sorry, I don't know anyone who has ever studied the effects of different brighteners and grain structures in resisting developing an "aged but still bright" look when exposed to various ferric chloride oxidizing agents. All I can do when you say that plating of a certain vintage works best is to try to refer you to literature describing the common platings of that vintage. I also did not realize you are trying to avoid buffing.
But you are misunderstanding some things too. Whether you pull a 'bright nickel' formulation from Wikipedia or from this site it does not mean that it will plate bright without brighteners and without buffing ... it means if you are going for a bright look, this is the basic Watts chemistry you can use to which you will add brighteners. Please try to look at the chapter on nickel plating in that ASM Metals Handbook and maybe try the formaldehyde and saccharin brightener combination that it speaks of.
Another thing I'm trying to explain is that people don't add quantities of brighteners and other tweaks to their whole tank full of solution based on book knowledge; that often leads to a tank full of witches brew. Instead, you experiment with small quantities and then scale up. Try to do one piece in a small portion of your solution to which you've added a small amount of the brightener combination.
2-10 A/Dm2 means per square decimeter, but you can work in English units if you prefer. I asked you how big the piece is: multiply the length x the width x 2 sides for an approximation of the square inches. Then divide x 144 to get square feet. Then multiply by the suggested A/ft2. But, yes, 2 Amps is probably the right ballpark.
Also please read up on "Hull Cell". I know you don't have one but it explains an orderly way to proceed in trying to develop a process, and it illustrates that if everything is right, the current density will not be knife-edge critical. For that reason you might try plating at 1 Amp/ft2. Another thing it gets across is that these pickup covers (if that's what they are) are probably not cheap, but you can develop your process with test panels rather than with difficult to replace components. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 2020
![]() I forgot I had that ASM book in PDF. A quick look mentions formaldehyde and saccharin but not in combination. But I only looked real fast to see if there is some simple to concoct brightener that comes out shiny, not dull. I did buy some saccharin, but I figured by itself it wouldn't do anything.
I forgot I had that ASM book in PDF. A quick look mentions formaldehyde and saccharin but not in combination. But I only looked real fast to see if there is some simple to concoct brightener that comes out shiny, not dull. I did buy some saccharin, but I figured by itself it wouldn't do anything.
Probably what I am asking is a brightener that doesn't really HARDEN the nickel so that it resists harsh environments. Mostly likely am looking for a brightener that leaves the nickel pretty soft, and is a super simple short chemical list. I saw a guy on youtube plate a penny with a simple Watts mix but his penny came out shiny nickel.
I've emailed a plating supply company and asked if they have some simple prepared mix for soft bright nickel, hopefully they can help.
I've even tried using my hobby bright nickel bath without ever replenishing the brightener and it just never loses the hard bright look. I bought another mix and just put in a tiny amount of brightener and didn't get a softer plate either. I'll keep looking and will look into that Hulls' Cell thing. Thanks again,
Dave
- BATTLEGROUND, Washington, USA
April 6, 2020
9th Edition, Vol. 5
"Surface Cleaning, Finishing & Coating"

on eBay or Amazon
or AbeBooks
(affil link)
"As I have said, please try to at least look at the chapter on nickel plating in that ASM Metals Handbook and maybe try the formaldehyde and saccharin brightener combination that it speaks of."
Q. I have the ASM handbook in pdf form, and there is no formaldehyde/saccharin brightener formula that I could find. I do dig thru these ASM and Nickel Plater's Handbook, over and over and over...
Do you have a page number reference? Or are you remembering from a different book? The only mentions of formaldehyde are for formaldehyde chloral hydrate, and I can't find anyplace that sells that, Fischer doesn't. Will just regular formaldehyde work combined with saccharin? I already have saccharin. Just need a super simple brightener so that after mirror polishing the nickel silver cover, it will come out plated shiny instead of matte nickel. As for your not understanding what I am trying to get, I want a soft bright plating right out of the bath with no polishing needed. The hobby stuff I use puts a HARD bright finish that resists all forms of chemical darkeners, "aging" acids etc. I also looked through the Nickel Plating Handbook as well. If it's not possible to make a simple easy to make brightener for a Watts bath, just give me the brutal TRUTH ;-) At least I successfully made the Watt's bath, and for that I thank you ;-)
- BATTLEGROUND, Washington, USA
April 9, 2020
A. Hi.
Nickel brighteners pp. 204-205
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 9, 2020
Q. OK, so if that makes sense. After reading some different places and sources, and looking on Fischer Scientific, would this get me where I want to go?
Carrier Brighthener
benzene sulphonic acid) in concentration 0.1-3 oz/gal (0.75-23 g/l)
Levelers, second class brighteners allyl sulfonic acid in concentration 0.0006-0.02 oz/gal
These are chemicals I can actually find and BUY.
Sorry to be so bothersome, but this stuff is slow to absorb, am doing my best. This involves spending another $70 probably, but looks worth a try? And yes I'd try it in a small sample of my Watt's bath.
Dave
- BATTLEGROUND, Washington, USA
July 3, 2020
Q. Am back again. I've decided to give up on coming up with a super simple bright nickel plating bath. So, I did buy these chemicals, saccharin, Benezenesulfonic, and Phenosafranin. I used suggested amounts per gallon and mixed it up and dissolved it in some distilled water and mixed about 70% of that into my perfectly working Watts bath that has no other additives. This mix was red in color, probably from the Phenosafranin. I got pretty excited when I saw down into the depths that the nickel was going on BRIGHT! FINALLY!!
But then I noticed some bubble adherence and wiggled the cover in the solution and bubbles came up. Then I noticed with a strong flashlight there was an aerosol mist coming off the top of the solution, directly over the plating nickel silver cover. Not good ... So, I let it run the full 30 minutes, I tried reducing the amps and it still was gassing off. But then even after the electricity was shut OFF, it was still creating bubbles off the cover. Bad news. When I pulled the cover out of the bath, it was evenly peppered with pin prick pits all over it. FAILURE.
Tried to save it by polishing it down, but I had to polish so deep, the plating was polished off. Pretty much this entire batch of over $100 in chemicals is ruined. I just wish there was a simple bright nickel bath without any hard chromium or whatever they use in the hobby commercial brighteners was printed somewhere, that you can mix yourself with simple chemicals that can be used in a Watt's bath. I looked and looked there is no such thing. Not even in the history of nickel plating did I find something like that.
I will make another Watt's bath, even though I have to polish it after plating, it did some things in my aging work that the commercial plating bath can't do, so at least I got something useful out of this madness. Photo sent for you to see:

I do kinda wonder if just the Phenosafranin by itself added to the Watt's bath would work, but I'm not rich ;-) I do have another obtuse question, I am using the blue polish from my supplier and have tried the white also, but no matter what I use, I can get a bright mirror finish, but there is alway these micro smear looking mark in the nickel plate. They say use talcum powder on the buff, but that sure doesn't work either. With the commercial plating bath I use it comes out of the bath not needing to be polished, but I wonder if there's a way to get a perfect total mirror flawless shine on nickel using a polishing machine if needed? Thanks for you time and help,
Dave Stephens
- BATTLEGROUND, Washington USA
A. Hi again. Personally I've never even heard of phenosafranine although google tells me it's a red stain used in biology.
Look at these pits and any previous pits with a jeweler's loupe or strong magnifying glass. If they are semi-hemispherical and bright they are hydrogen pits ("gas pits"). Although there are other potential causes, the chief cause of gas pits is lack of agitation and/or a solution that is not "wet" enough -- with the result that as hydrogen bubbles start forming they stick and the nickel plating grows around the bubble ... and that seems to be the situation that you are describing.
You need a wetting agent, sodium lauryl sulphate, sodium laureth sulphate, or similar: basically a few drops of shampoo. And you need agitation. You'll get tired of tapping the wire the part is hanging from every few seconds for half an hour to dislodge bubbles, but it's something you need to try.
But I tire of hearing about expensive parts being ruined and the cost of the vats full of chemicals, and struggling to try to peer into the solution with a strong flashlight when I've suggested experimenting with scrap or test panels and small quantities of solution instead :-)
Don't turn this into a moonshot where you must fly blind, suffer periods of non communication, and go for all or nothing with your vat full of solution. Instead, plate one small piece of scrap in a glass beaker [beakers on eBay or Amazon [affil link] so you can see what's happening without peering down with a strong flashlight and so that if your experimental addition of shampoo harms rather than helps the solution, it harms a liter or less (the Hull Cell that professionals use is just over 1/4 liter) not the whole volume. Make sure the contact wire is tight so it's not make-and-break, then tap the suspension wire, or tap the whole beaker up and down on the table to keep the bubbles dislodged and see if you can solve the "gas pit" problem. One problem at a time.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
July 2020
Q. They are gas bubbles for sure. I do have agitation, there is a small aquarium flow/filter pump that swirls the water slowly around in the pail. I jiggled the copper wire holding the cover to see if there was gas bubble build up because I couldn't see the flat surface under the dark green solution, and there was. No matter how low I ran the amps. Even with the electricity OFF there were bubbles building up on the cover, which I don't understand why, unless the pH got radically altered, which I have yet to check.
I did think about Sodium laurel sulphate, so I have not thrown the bath out yet. Maybe it will help, and I WILL give it a try. The Phenosafranin is from one of your recommended resource the ASM book or the Nickel plating handbook. In fact it was the ONLY brightener I could even FIND. Most of the ones mentioned just aren't available anywhere or have slightly different names than the simple reference names in those writings. Or they are illegal. Phenosafranin definitely WORKS. And yes it's very red which was a shock. I got my chemicals from Fisher Scientific. I did think about the Hull cell thing, but I'm not sure it would have caught this problem or not. I just crossed my fingers and made sure of the concentrations, etc. I'll give the laurel sulphate a try. I just checked the pH and its 3.4 pH, so at least that's not the problem. Thanks as usual, will see if the shampoo ingredient could solve this problem except I'll buy it pure instead of using shampoo ... Thanks.
Dave Stephens [returning]- BATTLEGROUND, Washington USA
July 3, 2020
Q, A, or Comment on THIS thread -or- Start a NEW Thread