-----
Hull Cell: What is it, How & Why to use it, and Much More
Quickstart:
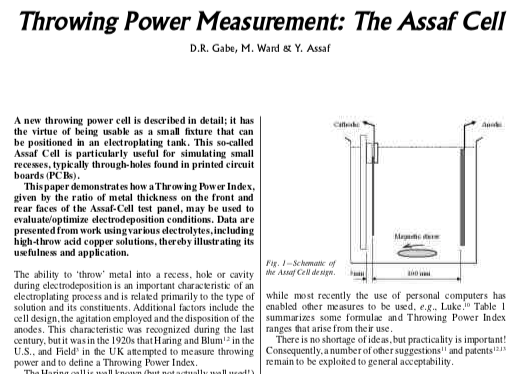
A Hull Cell is a tiny (usually 267 ml) electroplating tank for test panels rather than actual parts. It is trapezoidal, so the test panel is close to the anode
⇦ huh? at one end and far from it at the other end.

(courtesy of chemists-chemistry.com)
This shows the effect of plating at different current densities ⇦ huh? , helping the user figure out what is wrong with the plating solution or what needs adjustment. The size of the cell was chosen to simplify "scaling up": a 2 gram addition to the cell equals a 1 oz/gallon add to the main tank.
This thread attempts to cover every imaginable Q&A about theory, construction, options, and availability; but interpretation of results depends on what plating solution is being discussed, so please search the site for the plating solution in question plus 'hull cell'.
< Prev. page (You're on the last page of the thread)
Variations: Hanging Hull Cell
RFQ: We are looking for a Hanging Hull Cell. We have many in our shop that we purchased from McGean & Rocho, but they are no longer in the business. Please help if you have any suggestions for new or used equipment. Thank you.
Mary [last name deleted for privacy by Editor]Plating shop - West Springfield, Massachusetts
2003
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
Variations: Jiggle Cells
Q. I would like to know what a jiggle cell is and what information is obtained from it. Which companies make the equipment? Any help would be appreciated.
Robert Kestler- Cherry Point, North Carolina
1998
A. Jiggle cell is a 1000 ml cell that like Hull Cell ⇦ huh? enables to examine in lab conditions of plating solutions. It is a bent panel that gives more info on solution then Hull Cell, mainly on low current densities and roughness.
The only manufacturer I know is Mcgean-Rohco.

Sara Michaeli
Tel-Aviv-Yafo, Israel
A. I have used jiggle cells, but I have not found them to be of much more value than the Hull Cell. The cell is a tall, tipsy, contraption which moves the panel up and down. You bend the cathode into an -L- or a -b-. Then after you plate it, open up the closed area to view the extreme low current density areas. You can see as much by looking at the back of a plated Hull Cell panel. Shelf roughness is something you supposedly can check, but anode bags only work when you don't disturb them. Nobody lifts anode bags out a solution, and then expects smooth plating.

Tom Pullizzi
Falls Township, Pennsylvania
A. We used the Jiggle Cell and it is the best way to test some plating solutions, mainly Copper Acid and Fe/ZnFe... it show us the low current density areas, as the Tom says.
Assis, Silvio R- São Paulo, SP, Brazil
July 6, 2009
Variations: 1000 ml Hull Cells vs. 267 ml
Estimados Srs.
Estoy realizando algunas prubas preliminares con una celda de hull.
Consulta :
1) necesito las exactas dimenciones de la celda hull
2) Ecuaciones que relacionan distancia con densidad de corriente.
3) Publicaciones sobre celdas de hull para estudio de aditivos en EW de cobre.
Atentamente
Peter HolmesHull cell - Arica, I reguon, Chile
2004
A. Hi Peter. Public forums don't work well if people can't read the entries; please do your best to post in English on this one.
You'll see small variations in different reference works, but per C. Boulanger in "Investigation of optimal conditions for zinc electrowinning from aqueous sulfuric acid electrolytes", here are the dimensions of the 267 ml cell:
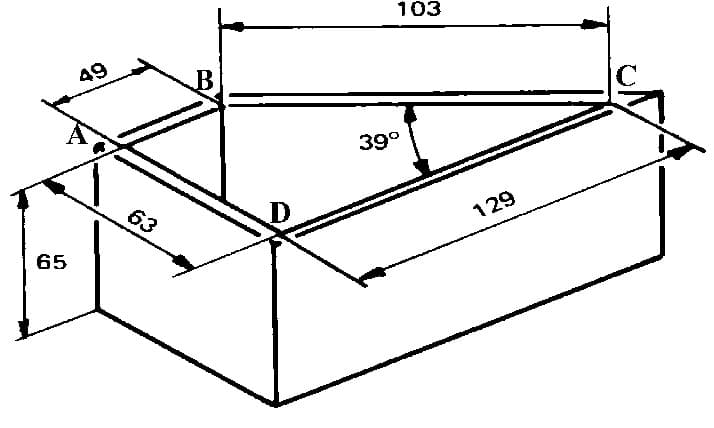
Your other questions are answered elsewhere in this thread.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Is it possible to supply me with the inside dimensions (millimeters) of the 1000 ml Hull Cell? Sincerely Tommy
Tommy van Rensburg- Vanderbijlpark, Gauteng, RSA
2004
A. Hi Tommy.
The 1000 ml Hull Cell uses a larger 5" x 3-1/4" test panel. In addition it squared off to a rectangular shape with that extra triangular space behind the test panel used, apparently, for extra volume. But sorry I don't know the dimensions.
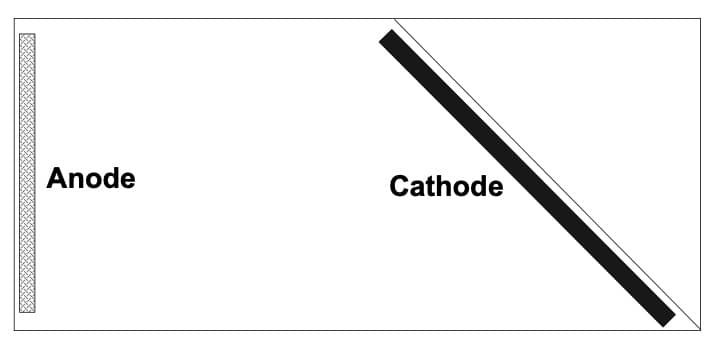
(from alertsales.com/wp-content/uploads/2020/08/Hull_Cell_Plating_Tests.pdf
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. THE STANDARD HULL CELL PANEL IS 75MM X 100 MM LONG BUT MANY OTHERS SIZES ALSO EXIST.
70 X 100, 70 X 200, 37 X 150 TO NAME A FEW
OSSIAN - Cork
May 27, 2008
A. When using Hull Cell the volume utilised is quite small and depletion of metal and or brighteners occur at a surprisingly high rate.
It's a good idea to use a perforated cell in a larger volume of electrolyte so that the electrolyte remains relatively constant.

Mel Platt
geriatic mel - Maidenhead, U.K.
November 11, 2008
Specs for Test Panels
Q. Dear Sir,
I want to know the standard for the brass test sheet for the Hull Cell ⇦ huh? test, especially the brass sheet copper composition.
Best Regards
Rae Wangpurchase management - Shenzhen, Guangdong Prov. China
December 15, 2008
Hull Cells for Chrome Plating
Q. Hi, I don't see anything on this thread about chrome plating hull cells. I have a chromic acid plating bath that I would like to track with a hull cell from time to time. We have one onsite, it's poorly constructed, doesn't seem to be a standard size (267 mL, for example), and doesn't have a heater. It's made of porcelain, I believe. Are there any other materials that can be used for chromic acid? Can you recommend a chromic acid hull cell? It would also be helpful to be able to heat the solution in the hull cell.
I am also wondering what the best settings for running a chrome hull cell would be -- run at room temperature for consistency? 5 amps? 10 minutes?
- Eau Claire Wisconsin
October 20, 2020
A. Hi Paul. I have no hands-on experience with hull cell testing for chrome plating, but you must run it at operating temperature, not room temperature. My understanding is that a perforated hull cell is suggested for chrome plating because of the otherwise fast temperature rise.
It seems that one approach is to hang a porcelain perforated cell in your chrome tank, whereas another approach suggested & offered by Mel Platt above, but also
www.yamamoto-ms.co.jp/en
is to place the 267 ml perforated hull cell into a 1000 ml enclosure tank -- I'm not sure of their material of construction, but not necessarily porcelain -- some plastics are sufficiently resistant to chrome.
Although there are guidelines for how long to run hull cells, in your case of decorative chrome where plating times are typically in the 30 sec to 2 min. range, I would think you would want to run it for the same amount of time as your plating time the first time around to get a feel for coverage; 5 A sounds right.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Hull Cell Rectifiers
I am looking for a copy of an operations manual, if there was one, for a Udylite PC-10 power supply (Hull Cell ⇦ huh? rectifier). We will be calibrating the power supply once a year for an ISO requirement and need the power supply specifications. Also, if anyone aready has a calibration procedure for the Udylite PC-10 and would like to share it, we would greatly appreciate it.
Joey Daniel- Lynchburg, Virginia
1999
Which Platers Need Hull Cells, Which Don't
RFQ: I am new in electroforming process and lately I encountered problems with nickel baths. I want to learn which methods can be used to test the contents (any contamination, etc.) of the baths and which information I can get by carrying out Hull Cell tests for my nickel sulfamate baths.
Does an electroformer really need that piece of equipment? Right now, by simple titration, I determine Ni++, Cl-, and boric acid concentrations of the bath. Any suggestions about those concentrations? And also any suggestions about Hull Cell test equipment suppliers in EU or USA? Thank you for your replies already.
Limec I [last name deleted for privacy by Editor]plating shop - Berlin, Germany
2005
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. Hi Limec. In the opinion of industry icons a lot smarter than me, everyone doing any plating needs a Hull Cell. Here are fout reasons that I can think of offhand.
Firstly, although this one may apply less strongly to nickel electroforming than other plating, a Hull Cell lets you see what will happen at very high and very low current density in case a part you are making will require plating in that range.
Secondly, it helps you spot trends before you make defects -- if the good plating range is declining from one Hull Cell test to the next, you have advance notice that the problem may soon extend into the current density range you are using.
Thirdly, some defects in the plating solution, like contaminants or lack of addition agents, can be identified by the fact that they cause poor plating at certain current densities more than others.
Fourthly, and this applies to everyone doing any kind of plating, you should never make additions to your tanks based on theoretical calculations. Rather, you should do the theoretical calculation, make adds to the Hull Cell ... and only after you have verified the results of the add do you go on to scale it up to bath size.
Please post your questions about nickel sulfamate plating under one of our threads about that topic. Sorry for the inconvenience but when threads veer from their main topic, readers can't find stuff.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
The mathematics of Hull Cells
Q. For Hull Cell I need an equation to find current density at different distances of cathode.
HECTOR LONGUEIRA- ANTOFAGASTA, II region, CHILE
2004
A. Hi Hector,
This is the formula to calculate current density for a Hull Cell panel.
c=i(5.1-5.25logx)
c= current density
i= current applied
x= distance to be measured on the panel (panel side close to anode is HCD area and side away from anode is LCD area)
- Kitchener, Ontario, Canada
Ed. note: The result of this calculation is displayed on a Hull Cell Ruler.
Q. Dear sir/ma'm
I am pursuing research in alloy electrodeposition. In this regard I would like to know the theory of 267 cc Hull cell equation c = I(5.1-5.24logx) in terms of either simple mathematical simulation or trigonometrically.
Research scholar - Rajapalayam, Tamilnadu & India
September 15, 2008
A. The Hull Cell was invented by RO Hull and a paper was published in 1939 by him about it. I suggest you track down this paper as it may well describe the mathematics behind the system. There is also a book called The Hull Cell by Walter Nohse [on
eBay in English,
Amazon in English, &
AbeBooks in original German affil links]
; this was published in 1966 by Robert Draper, Teddington. Again this may well contain the math you require.
The actual math is quite easy to work out once you have understood the principles of the cell, but it is made even easier by computational modeling; so if you cannot track down the above texts, you may want to attempt solving it yourself.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
A. Try this site:
www.springerlink.com/content/x118154920626732/ ⇩
for "Primary current distribution in the Hull cell and related trapezoidal geometries". Quite possibly deeper than you need.
Jim
- Navarre, Florida
Ed. note Oct 2020: That sprinkerlink.com domain no longer exists, but the article is available at
link.springer.com/article/10.1007/BF01030192
for a $39.95 fee to the middlemen sitting between the uncompensated researchers and the starving students 🙂
... but ResearchGate.net is currently offering it for free at https://www.researchgate.net/publication/227203087_Primary_current_distribution_in_the_Hull_cell_and_related_trapezoidal_geometries/link/550bdba00cf265693cefb333/download
Q. Dear Sr. :
I have two questions about Hull cell :
Using the current density equation of the Hull cell ( C : I ( 5,1 - 5,25 log x) ), there are an infinite currents that might be used (usually described as 1, 2, and 3 A ). I Need to evaluate additives between 200 and 500 Ampere per square meter.
Q1 : Which current should I work with the hull cell.
Q2 : What time of deposition should be use.
Sincere regards.
Peter Holmes- Antofagasta, II Region, Chile
2004
A. Hi Peter. That's a very normal range for most production plating. Converting to English units because the Hull Cell rulers I see are in English units, it's 18.6 to 46.5 ASF ... and just about dead centered on the Hull Cell panel if you plate at 2 Amps.
Plating time really can't be estimated because zinc might be plated to 5 µ and nickel plated to 20 µ for example.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. It might help if you would tell us about the plating bath you want to evaluate ...

James Totter, CEF
- Tallahassee, Florida
A. Dear Mr. Holmes:
You need to be more specific in your question.
Hull Cell can be applied to many electroplating processes, with different purposes.
- Santiago, Chile
A. Applied current and testing time all depend on what you are testing in the Hull Cell. There are set conditions for different plating systems, details of which can be found in any reputable book that discusses the Hull Cell. Try The Canning Handbook [on eBay, Amazon, AbeBooks affil links]. No one can answer your query unless you say what you are doing.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Hull cell modeling
Q. Dear sir,
I would like to know whether any mathematical modeling model is proposed for alloy plating based on Hull Cell
⇦ huh?
.
Mohamed Sathak Engineering College - Kilakarai, Tamilnadu, India
2005
A. I am not really sure what you are asking, but I suspect you have been asked to use a Hull cell to determine the plating characteristics of an alloy system. A Hull Cell can be mathematically modeled as the local current density on the working electrode is related to the geometry of it (the working electrode) relative to the auxiliary electrode. However, when plating an alloy, its composition will be dependent on the applied current density. Therefore, by using a Hull cell, you can controllably alter the alloy composition across the working electrode surface. The composition can therefore be modeled once you know your operating parameters and the properties of the alloy.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Q. Hi to all the experts of electroplating. I need your help regarding electroplating speed of an electrolyte: please tell me how to calculate the plating speed of a electrolyte using the standard Hull Cell ⇦ huh? panel?
Veerendra kumar- Delhi, India
June 26, 2017
A. Hi Veerendra
A Hull cell is not used to measure plating speed.
Its principle use is to determine the optimum range of current density or to diagnose problems with organic additives, etc.
Use your hull cell to find the best operating current density; then plate a sample panel of known surface area at this CD and measure the thickness.
Don't be tempted to run the process at the absolute maximum current density, however tempting to reduce the process time, as it will be more sensitive to small variations in the process.

Geoff Smith
Hampshire, England
July 9, 2017
The Hull Cell Ruler
Q. How do you read the hull cell ruler?
⇦ on
Amazon [affil link] It is stated from the ruler that it has 1, 3, 5 Amps. how much time should you use? 1 min or more?
We are currently using SnPb chemistry for IC packaging. I'm a bit confused as to how to read the ruler. I'm currently using 3 Amps 2 min. when I read the result using the ruler, I use the 3 amp portion and multiply the Current Density by 2 -- is this right? We are using 10-20 ASD meco plating machine
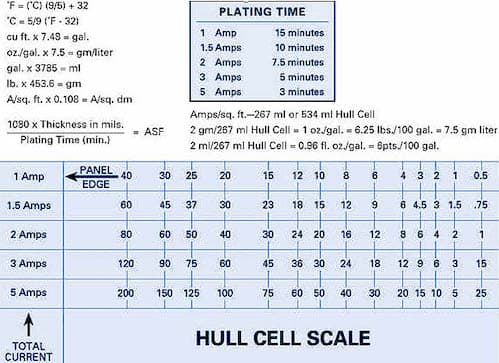
(graphic courtesy of nusourceonline.com)
I hope you guys can answer this question the soonest. Thanks in advance!
Michael Tongson2005
semiconductors - Laguna, Philippines
A. Hello,
Hull-cell current to be employed depends on the type of plating you are investigating and the average current density and maximum operating current density you are likely to encounter in the production tank.The purpose of the Hull cell experiment is to know the plating quality at different current densities in single test. Generally, for vat plating it is customary to use 5 amps and 10 minutes to get the test result. 5 amps will give a range of 0-200 amps/sq. ft current density.This range, I think, will be sufficient for most of the decorative or commercially useful applications.
Good Luck!

Subramanian Ramajayam
consultant - Bangalore, India
December 19, 2011
How to Know Current Density in the Actual Tank?
Q. Hi all,
I've been using a hull cell to evaluate the best plating parameters for a sulfuric acid copper chemistry. I now have the ideal additive ratio and current density but how do I figure out the current density of my piece in my larger plating bath. Also in my bath the anode-cathode separation is much larger than the hull cell.
Is there a rough equation for determining the current density at a point x distance from the cathode? Or do I just have to play with the settings?
Any help appreciated,
Thanks,
David
Student - Ireland
September 22, 2008
A. Hi, David. Figuring the average current density should be no trick. You just divide the total amperage by the surface area of the component, and it doesn't matter what the anode to cathode spacing is. The current density will vary across the component -- highest at edges and corners, lowest in the middle of expanses. Anode to cathode distance matters for this, but probably not at much as the geometry of the part. Software is available for modeling current density across a part but it's not a trivial calculation and more likely involves finite element analysis.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. The Hull Cell is an excellent tool for determining how an electrolyte acts under different current densities. The current density at any particular point on the Hull Cell plate will be dependent on the current applied to the cell, and there are standard Hull Cell tests that will tell you these values. When transferring this information to a real plating cell, the difference is that the anode-cathode distance should be the same for as much of the plating part as possible, otherwise you will get differential plating rates. Once you know the optimum current density for plating from your Hull Cell test and have calculated the surface area of your plating part in your larger tank, you can work out the total current you need to apply to your part. In practise, the greater the anode-cathode distance, the better the thickness distribution will be.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
November 13, 2008
Q. Hello Sir,
I am doing my deposition of copper using Comsol Software. How can we convert the current intensity in terms of dm^2 ?
- Furtwangen, Germany
April 15, 2012
Ed. note: More explanation please, Lohith, I don't understand the question.
Doing a Hull Cell Research Project
Q. Hello,
I am a student in chemical engineering and I am doing research on Hull Cell Testing.
And I need information on how it works, like the operative methods, and how to analyse the results, so if you can write it to me or give me some links about it, I will be very thankful.
Thank you
- Paris, France
April 23, 2014
A. Hello Jack. We appended your inquiry to a long and interesting thread on the subject. So please read it and think about what you would like to research in particular, then ask a specific question and we'll try to help. Good luck!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Using a Hull Cell for Plating Actual Small Parts
Q. In a Hull Cell can I use as a cathode a part (not flat) that needs to be coated?
Julia Savchenko- Austin, Texas, USA
March 28, 2017
A. Hi Julia. Sorry, no, a Hull Cell is terrible as a general plating tank :-(
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. The part that I need to coat is magnet NdFeB; can I use cathode made out of NdFeB in a Hull Cell or in a Rotating Cylindrical Hull Cell? Thank you!
Julia Savchenko [returning]- Austin, Texas, USA
A. Hi again, Julia. I guess you're not asking about a hull cell as a testing device but as a small general purpose electroplating tank for your magnet.
It's not ideal for that for a few reasons, including the fact that a hull cell is designed to plate only one side of an item rather than both sides. I think you'd be much better off using a glass beaker [beakers on
eBay
or
Amazon [affil link] so you can put an anode on each side of the part and so you can easily use a hotplate/magnetic stirrer
⇦ on
eBay or
Amazon [affil link]
for agitation.
But alloy plating is very difficult, and your chances of electrodepositing NdFeB sound lower than of winning the Powerball lottery. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Thank you very much for taking the time to answer my questions.
Thank you very much for taking the time to answer my questions.
Urban Mining - Austin, Texas, USA
A. I would use a plastic polyethylene shoebox as a plating tank.
I'm not clear on what is being plated here. I was plating Cu and I used 3/8" copper tubing as anodes. I flattened little sections at one end, drilled holes, and joined them to the mickey mouse "busbar" with brass screws. Worked fine.
Ni would be tougher. Anodes there are generally Ni "crowns" and you would have to have some kind of conductive anode basket to contain them.

Dave Wichern
Consultant - The Bronx, New York
Other Plating Cells Besides Hull Cells
Q. Hello, I'm about to start an experiment about analysing the thickness distribution of an electrolytic bath, I'm looking for information about different cells that I could use in the lab, but can't seem to find information about cells other than Hull. Does anybody know where I can find this kind of information, or suggest any cell suitable for this experiment?
Thank you in advance.
- Sonora, Mexico
November 1, 2017
A. Hi Ricardo, there are many styles of Hull Cells including perforated hanging hull cells (for use within a plating tank), double size (534 ml) cells, 1000 ml cells, and "jiggle cells".
But there is also a different kind of test cell called a Haring (search the site) or Haring-Blum cell which is used to measure throwing power, and which can be used for thickness distribution measurements as well.
But it seems to me that a Hull Cell is the ideal tool for thickness distribution determinations. There are youtube videos specifically about this; here is one courtesy of EPI / Electrochemical Products Inc. [a finishing.com supporting advertiser]...
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello everyone!
i was wondering if is there a way to measure or determine the Throwing Power Index of my electroplating solution with a Hull Cell or what's the specification to develop my own Haring Blum Cell??
Thanks in advance
-Sinaloa México
December 4, 2021
A. Hi Jorge. To the best of my knowledge the Haring Blum Cell has no special dimensions, just the ability of the anode on one side of the cathode to be 5X as far from the cathode as the anode on the other side. The article "Throwing Power Measurement: The Assaf Cell" ⇨
gives some good history and formulas.
But yes, you can calculate throwing power from thickness measurements on a Hull Cell panel. EPI offers a youtube video on how to do it.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Some Practical Calculations
Q. Hello,
I have a Hull cell in my lab (around 267 ml cell, I think). I am trying to perform Hull cell tests with some nickel baths. My Hull cell plate has an immersed surface of 50 cm2 (5 x 10 cm). My bath works at 1.5 A/dm2. How many amperes should I use : 1.5 x 0.5 = 0.75 A. Is it correct? I have seen that we should use 1, 2 or 5 A with use cell (as noted in many hull cell ruler). What is right?
Another question, I have forgotten my Hull cell ruler
⇦ on
Amazon [affil link] . Is it possible to draw/create my own Hull cell ruler? How to do it? Could you help me please?
Thank you in advance
Best Regards
Thomas
- Berne, Switzerland
May 25, 2020
A. Hi Thomas. The Hull Cell is deliberately designed to exceed reasonable values at both the high current density and low current density ends of the panel, so I don't know that it's appropriate for what you are trying to do. But if you want to plate at 1.5 A/dm2 (13.4 ASF), what you actually want to do is find 13.4 near the middle of a Hull Cell ruler, which you will on the 1 Amp scale. So plate at 1 Amp.
adv.
Download the EPI Finishing Utility app and you'll have your ruler, plus other stuff :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello,
Thank you Ted for your reply! This app looks great but unfortunately impossible to download it on my phone or tablet: This app is not available in our country :(
I will try to construct my own Hull scale. If someone can help me to construct my Hull scale, let me know please. I need to know the current density distribution on the Hull cell pate. And about the current to use, can we use whatever we want or is it advise to use 1, 2 or 5 A as specified in the Hull cell ruler?
Thank you in advance
- Berne, Switzerland
A. Go to google.com - images - and search for hull cell ruler pdf and you will get a lot of choices. Even in the restrictive climate in the EU that shouldn't be a problem.

Tom Rochester
CTO - Jackson, Michigan, USA
Plating Systems & Technologies, Inc.

Q. Hello,
Thank you Tom for your suggestion! Good idea, I had not thought about it!
I saw this formula in this thread :
c=i(5.1-5.25logx)
c= current density
i= current applied
x= distance to be measured on the panel (panel side close to anode is HCD area and side away from anode is LCD area)
What are the units in this formula?
Do you think I can use it to construct my own Hull cell ruler? But with this formula, current density will be infinity at x = 0, possible??
Thank you,
Best Regards
- Berne, Switzerland
A. Hi Thomas. I don't have the reference which offers that equation, but it seems to be more of a "curve-fitting" formula than a derivation based on first principles.
Current density is most often expressed in A/dm2, and it must be in this case because were it measured in A/m2 or A/cm2 the current density would be 100X lower or 100X higher, and that sounds unreasonable.
Since a Hull Cell panel is 10 cm long, and log10 = 1, x must be measured in centimeters. At a current of 1 amp then,
CD at 1 cm from HCD end is 5.10 A/dm2
CD at 5 cm from HCD end is 1.43 A/dm2
CD at 8 cm from HCD end is 0.36 A/dm2
CD at 9 cm from HCD end is 0.09 A/dm2.
My limited understanding is that the formula is considered valid and usable only between 1 and 8 cm from the HCD end, so the fact that log0 is undefined and approaches negative infinity doesn't matter.
Luck and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Hello,
Hello,
Thank you Ted for your comments! I will try to construct my own Hull cell ruler with this formula.
Best Reagards
- Berne, Switzerland
Ed. note: ... or just print one out at the proper scale (see 'Hull Cell Ruler' on this page.
Q, A, or Comment on THIS thread -or- Start a NEW Thread