Home of the world famous 'finishing.com HOTLINE' (since 1989)
-----
Hull Cell: What is it, How & Why to use it, and Much More
Quickstart:
A Hull Cell is a tiny (usually 267 ml) electroplating tank for test panels rather than actual parts. It is trapezoidal, so the test panel is close to the anode
⇦ huh? at one end and far from it at the other end.

(courtesy of chemists-chemistry.com)
This shows the effect of plating at different current densities ⇦ huh? , helping the user figure out what is wrong with the plating solution or what needs adjustment. The size of the cell was chosen to simplify "scaling up": a 2 gram addition to the cell equals a 1 oz/gallon add to the main tank.
This thread attempts to cover every imaginable Q&A about theory, construction, options, and availability; but interpretation of results depends on what plating solution is being discussed, so please search the site for the plating solution in question plus 'hull cell'.
Books and Technical Articles about Hull Cells
Q. Does anyone have the ISBN code for R.O. Hull's "little book"?
John Persaud- Reading, UK
2000
Ed. update: decades later we've never found that particular book; but this thread covers other books and many other resources.
Q. I want to buy a book about Hull Cell ⇦ huh? test, do you have any suggestion to give me?
Joaquim AlmeidaFriedrich Grohe - Portugal
2001
A. The American Electroplaters and Surfaces Finishers Society used to have an excellent "Illustrated Lecture" (booklet plus 35mm slides). Try www.nasf.org to see if it's still available, perhaps as a DVD.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Ed. note: Read on please; the thread now references a book plus countless other resources about Hull Cells.
Q. I am looking for a book and a booklet. I would appreciate any information that anyone has about either or both of these publications.
1. The book is called "The Hull Cell." I think it was written by a woman. It had a lot of tips and instructions for using the Hull Cell. It even had a Hull Cell ruler bound into the spine of the book on a piece of ribbon!
2. The second is a booklet published by a vendor. I don't remember the vendor, but the booklet was a very nice presentation about the history of the beginnings of electroplating. It has a glossy white cover and is called "From Faraday to Frog Legs" There are short paragraphs on Volta, Ampere, Sir Humphrey Davies and Michael Faraday. The booklet is illustrated with really cute line drawings. Any and all help is greatly appreciated.
Regards,
Sue Jones- Canyon Country, California
2002
A. Marilyn K. Sanicky worked for McGean Rohco and did a series of articles on the Hull Cell. I think they were carried in Metal Finishing. Perhaps they can sell you copies of the series. It was very good! She has retired from McGean (which may have since merged into something else).
Jon Quirt- Fridley, Minnesota
A. Hi Sue. Marilyn [dec.] was the author of a series of plating journal articles entitled "All about the Hull Cell" as well as the illustrated slide lecture offered by AESF entitled "The Use of the Hull Cell"; it is possible that an AESF/NASF branch has a copy of that illustrated lecture (I had a copy but lost it to Superstorm Sandy).
"The Hull Cell" was written by H.J.Sedusky & J.B.Mohler, but WorldCat.org shows no copies of it (we're now thinking this was just a paper, not a book).
That Faraday & Frog Legs book was published by Hanson-VanWinkle-Munning in 1997; through worldcat.org I'm finding one copy in the world -- available at Wayne State University library in Detroit, and they still have it, ref.:
elibrary.wayne.edu/record=b2152282~S47
Atotech has put together a paper based on, and incorporating, Marilyn's work -- which I think the readers will find helpful; unfortunately it offers no photos of hull cell panels ⇨
but it references the H.J.Sedusky & J.B.Mohler work, so someone somewhere has a copy 🙂
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Updated May 2025
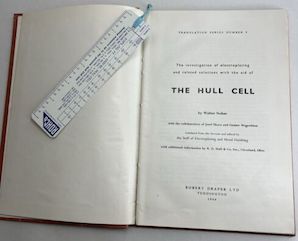
A. "The Hull Cell" must have been a very popular title. There is a book of this title by Walter Nohse ⇨ published by Robert Draper of Teddington UK in 1966, ⇨
It was translated from the original text by staff of "Electroplating and Metal Finishing" .
I cannot vouch for the cover, as I haven't seen one with a fly cover intact ⇨
Ed. note: See Sjamp Van Esch's reply below regarding the original German version.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Q. Our company is one of the largest Electroplating Rectifier manufacturers and exporters in Turkey. I am searching for documents about Hull Cell, to give enough information to electroplaters, (especially Sedusky & Mohler's "The Hull Cell" article, R.O. Technical Bulletin No. 404), but cannot find any except McGean-Rohco Inc.'s article dated 08/1997. I also want to buy a detailed book about Hull Cell Test [on eBay in English, Amazon in English, & AbeBooks in original German affil links] Please help me about these two matters. Where can I get that article and the book from? Already now thanks for your aid.
Alper Onsavas- Istanbul, Turkey
2003
A. Maybe this book is something for you: "Die Untersuchung galvanischer Bader in der Hull-Zelle", it's written by Walter Nohse, ISBN-Nr 3-87480-016-4. You can order via internet from the publisher "Leuze Verlag" www.leuze-verlag.de
Kind regards,

Sjamp van Esch
- Eindhoven, The Netherlands
R.O. Hull's granddaughter chimes in!
A. I am responding to Mr. Alper Onsavas of Istanbul, Turkey (2003) who was interested in articles about the Hull Cell. Mr. Alper, did you find the articles you needed? My grandfather, Richard O. Hull was the inventor of the Hull Cell and I would be most happy to provide you with needed research information about the Hull Cell, if you still are in need of same.
Kathleen A. Hull- Westfield, New Jersey, U.S. A.
Q. Kathleen Hull, mind if you can discuss to me how the hull cell scale (or ruler) is used and how can we interpret the result ... how do you determine which part of the brass panel is within the boundary of our plating specs? we are using 10 - 30 ASD for our plating line. Also, I can't seem to figure out how much current and how long should I plate the brass panel we have ... any recommended amount of current and time?
thanks in advance!
Michael Andrew Tongsonsemiconductors - Laguna, Philippines
Q. Kathleen A. Hull,
I am in need of Hull Cell information and would greatly appreciate it if you would provide me with information as to where I may obtain this information. I am new to electroforming and am eager to learn. Thank you.
- Robbinsville, New Jersey, USA
2007
Q. I'd like to receive information on how to set up, use and interpret the results of a Hull Cell.
Daniel Doom- Wichita, Kansas, USA
2004
Q. Sir I would like to know the setup (construction parameters) , use, and how to interpret the results from plating using Hull Cell ⇦ huh? .
R.RAMYAstudent - Chennai, Tamil Nadu, India
2006
A. For Hull cell test you can see the links below:
https://www.thinktink.com/stack/volumes/volvi/hullcell2.htm
https://www.thinktink.com/stack/volumes/volvi/hullcell2.htm
- Tehran, Iran
Ed. note: Thanks Reza, those links are very helpful! (still working in May 2025)
Multiple threads merged: please forgive chronology errors and repetition 🙂
Purpose of the Hull Cell?
Q. What's the simplest meaning of Hull Cell test? And also its purpose?
Thank you very much in advance for the information.
Juliana Marie Bustamanteelectronics - Cavite, Philippines
2004
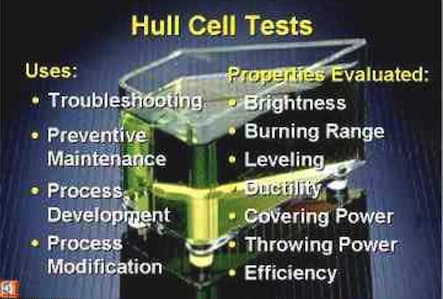
A. Hi Juliana. A Hull Cell is a miniature plating tank that is specially designed to assist the user in the analysis and troubleshooting of plating problems.
The simplest type is 267 ml in volume and a "right trapezoid" in shape from a bird's eye view. The anode ⇦ huh? plate and cathode ⇦ huh? test panel are placed along the non-parallel sides so that the current density on the test panel ranges all the way from lower than real parts would ever see to higher than real parts would ever see. You use it to find the 'bright range' of plating solutions (for example a proper nickel plating solution will be bright nearly from end to end, but a poorly adjusted solution will be cloudy in areas, or not give coverage). Solution adjustments can be tried out in the Hull Cell before being applied to the production tank, then 'scaled up' from the 267 ml to the size of the plating tank.
This presentation at nmfrc.org is a good intro ⇨

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. I'm a new employee and can't seem to find our manual for the Hull Cell. Can you please explain to me the procedure. How long should we plate the panel, how much current? If you can also give a website that has data on Hull Cell analysis, such as pictures of high metal contamination or low brighteners, etc. would really appreciate it. thanks!
Michae Andrew Tongsonsemiconductor - Cabuyao. Laguna
2004
A. Hi Michae. I doubt that you'll find such a website because the Hull Cell is such a versatile tool and is used for so many different plating solutions, and in such different ways for them -- but we appended your inquiry to a long thread on the topic. Tell us what metal you are plating please, or search our site for discussions of the plating solutions you have in mind. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. In the book "Modern Electroplating", author: Green,
you will find a good description of the principles of the two types of current distribution:
Primary and secondary. The primary is theoretical, based on the geometry of the cell with electrodes. The secondary is experimental, including the effects of the overvoltages at both electrodes. These overvoltages make the distribution more equal over the electrodes.
A third affect occurs when the current efficiencies depend on the current density.
These three effects together result in the effective product distribution over the surfaces.
advisor surface treatments - Eindhoven, the Netherlands
Ed. note: We find no reference to "Modern Electroplating" authored by "Green". Such a discussion can be found, however, in Lowenheim's "Modern Electroplating" ⇨
The cover is green 🙂 -- maybe this was a bad autocorrect?
Multiple threads merged: please forgive chronology errors and repetition 🙂
|
|
Need Hull Cells or Component PartsRFQ: I am looking for someone that can tell me where I can purchase a Hull cell. If you know of a company that I can contact, let me know. Thanks, packaging 1998 Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?). RFQ: I have been searching for information on manufacturers of Hull Cells. So far no luck. Can you help me ... thanks Stan B.Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?). RFQ: Please let us know who can supply in Argentina, Hull Cell or if you have got distributors here. Thank you. Carlos Rottgardt -- MUNRO, BUENOS AIRES Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?). RFQ: Please give specific supplier for Hull Cell equipment here in China. - Zhuhai, China 2005 RFQ: I am in need of a couple of cadmium hull cell anodes to fit a 267 ml hull cell. George P [last name deleted for privacy by Editor]Plating Chemical Supplier - Bedford, Ohio 2007 Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?). |
Ed. update Dec. 2023: The internet was much more limited back in 1998, more technical, far less commercial. Hull Cells are available from countless sources today, including eBay and Amazon ⇨
! Don't make big research . Every Plastic craftsman can do the job for you . Give him the exact sizes you need and he will do the job for you.

Yehuda Blau
YB Plating Engineering and Quality - Haifa Israel
![]() A. I don't know, Yehuda. It's looking like there might be less research involved in coming up with the name of a manufacturer than in trying to find the precise dimensions to use :-)
A. I don't know, Yehuda. It's looking like there might be less research involved in coming up with the name of a manufacturer than in trying to find the precise dimensions to use :-)

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. I agree with Ted, it is not a do it yourself job. We tried to do it on our own once and tiny differences in measurements of the cell produced big differences in results.

Sara Michaeli
Tel-Aviv-Yafo, Israel
![]() Apparently a 10 ml variation in electrolyte can make a vast difference to results. I made a 267 ml cell and achieved good quality plating from this.
Apparently a 10 ml variation in electrolyte can make a vast difference to results. I made a 267 ml cell and achieved good quality plating from this.
- Scotland
!! In linking this letter to another, I happened to read the responses, and Sarah's is the most interesting from a scientific perspective. It indicates, to me at least, that I should be careful when setting up a line to plate a new item. In other words, field trials of:
- racks
- tanks
- barrels
- anode placement
- distance of the part to the solution level and
- bottom and
- sides of the tank
- placement and proximity of heating and cooling coils and
- filter piping ...
... are necessary to make sure that you have the proper current distribution.
I would like to know more about the tolerance of the Hull Cell needed to make for accurate measurements. And does R.O. Hull's little book mention this problem?

Tom Pullizzi
Falls Township, Pennsylvania
A. Tom, I thought there is a German standard for the tolerances; I will look that up. In either case the Book of Walter Nohse about the Hull Cell method doesn't give any tolerance.
Bye,
Harry

Harry van der Zanden
consultant - Tilburg, Netherlands
|
|
Suppliers of Hull CellsA. There is a supplier in Taiwan called Giant Chemicals. Ricks Lam- Hong Kong 1998 A. Kocour in Chicago is another source of Hull Cells and related supplies.  Ken Lemke Burlington, Ontario, Canada A. Hull cells are supplied by Ecograph AG Switzerland khalid khanani- Pakistan A. I HAVE BEEN BUYING ALL HULL CELL EQUIPMENT FOR THE LAST 6 YEARS FROM PLATING TEST CELL OUT OF OHIO. JOHN ROLLINS- FRIDLEY Minnesota 1999 A. I have an address for you here in the States: Kocour Company 4800 South St. Louis Avenue Chicago, Illinois 60632 U.S.A. - Denmark 2000 A. Frank, Just for your information and anyone else who is interested I thought I would pass this on. It is correctly stated that you can get a Hull Cell from McGean-Rohco. I would like to point out to all that the Rohco in the name is the former R.O. HULL COMPANY the inventor of the Hull Cell, R.O. Hull. Hope this info is helpful for others in the future. Charlie Zipprich- Winston-Salem, North Carolina A. Try Larry King Corp. I did business with them for years. They sell the cells, the flat panel cathodes, and anodes.  Dave Wichern Consultant - The Bronx, New York March 29, 2017 Ed. Update: Please don't update this suppliers' list. |
[ed. note: this entry appended to a thread already addressing it]
RFQ: I want to buy Hull Cell Tester but I don't have any specs or part number at present; can you please assist?
Thanks,
- Clarkfield Pampanga, Philippines
2003
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. Hi May.
Most people are looking for is a standard 267 milliliter cell
⇦ on
eBay
or
Amazon [affil link] . You probably also need a small rectifier
⇦ huh? , and a heater to maintain correct plating temperature.
But there are also modifications like air agitation, hanging cells, jiggle cells, 1000 ml cells, etc. If you tell us what kind of plating you are doing, readers may be able to help you further regarding those specialties.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
(you are on the 1st page of the thread) Next page >
No dead threads!
Your Q, A, or Comment puts this thread on The Finishing.com HOTLINE.