
-----
Clear Anodizing Problems & Solutions
@Dave Demaree, I am still trying to wrap my head around why your parts are normally champagne color (mine are too, but I dye them black so no worries. I just assumed it was something funky in my process). The point is, I can't produce a true colorless "clear" anodize on 6061, though I have read that it's commonly done by commercial anodizers. Sooo, either 6061 doesn't actually anodize clear, or Dave and I have found a way to consistently get a champagne color that nobody else seems to get?
Larry papasmall scale anodizing of in-house parts. - portland, oregon USA
August 23, 2022
|
|
A. Hi Larry, supervisor - Cicero, Illinois Saporito Finishing Co.  A. Tell us the free acid, dissolved aluminum, temp, current density, and heat treatment of the 6061.  Robert H Probert Robert H Probert Technical Services Garner, North Carolina  |
Q. Thank you sir.
We have never seen the anodized surface come out clear, it's always got that slight champagne tint.
Here is the rap sheet detailing my crimes against anodizing...
Anodize parameters type II sulfuric :
20 gallon plastic tank with sheet aluminum for cathodes.
Tank temp: target is 70 but it can wander from 68 to74 without any noticeable problems.
Tank concentration: The Free acid and aluminum are adjusted weekly. 25% is decanted and replaced with fresh makeup of DI water and acid, according to what's needed.
Free acid: 20-23 oz/gal
Aluminum: 2.7-3.0 oz/gal
Target coating thickness 1 mil
Time in the bath 1 hour.
.1 ^ 0.1 amps per sq in. using constant current, which typically runs at 18 volts but varies with concentration levels.
Agitation is by acid pump, which also serves as the cooling loop.
Material is 6061-T6, some machined shapes and some extruded tubing.
Process sequence with rinses between:
-alkaline cleaner
-pre-smut
-etch
-de-smut
-anodize
-dye
-mid temp seal
The rinses are tap water except one DI water rinse prior to the dye bath.
We typically run 6 loads per day, most of it black. These parts are for exercise equipment and get direct sweat contact, so corrosion resistance is a high priority.
- Portland, Oregon
August 26, 2022
? Are the parts champagne color coming out of the anodize tank or after sealing? Some mid-temp seals can impart a slight color.
Q. If the parts look good coming out of the anodize tank you could try a hot DI water seal or high temp seal that advertises no color.
- Rock Hill, South Carolina
September 14, 2022
![]() David thanks for that.
David thanks for that.
Q. The color is present right out of the anodizing tank.
What's funny is over the years I have either cut corners or made mistakes that resulted in poor outcomes, but the color of the anodize layer right out of the anodizing tank has remained constant. That is why I'm so curious about the cause.
Larry
- Portland, Oregon
September 27, 2022
A. Two things to look into, first is that your dissolved aluminum content is pretty high. Most literature sites about 0.5-1.5 oz/gal (4-12 g/L) on dissolved aluminum content. May be worth your time to make up a fresh bath, just make sure to add back enough of the old bath to prevent burning. An option to test this theory would be to make up test cell in a 5 gallon bucket and coat a test panel. You can use frozen water bottles floating on the surface in a pinch to help control the temp as long as you had adequate solution circulation (I've used aquarium pumps in a pinch before).
Second thing, I would consider sending out a sample of your bath and make-up acid to a lab for metals analysis. Your make-up acid could have a metallic contaminant that is contributing to the color of the oxide layer (I'm thinking possibly zinc).
- Rock Hill, South Carolina
Q. Dave
Yes the aluminum is on the high end, but we have run on the lower end of the range without any change in the anodize color. I will admit we often let the aluminum go higher than we'd like, but I'm really not sure there is a downside. The parts always dye nicely, seal normally, and look great.
As for having our acid tested, I have to wonder what we could learn from that. The cathodes are basically dissolving during the process, so you'd almost expect to find other metals like zinc in the bath.
With that in mind, I will run a test bath of fresh acid just for good measure, but I want to try a higher grade of acid if I can find it. I think there is likely something funky about the acid we currently use, even though it comes from a plating supply house.
Thanks
Larry
- Portland, Oregon
⇩ Related postings, oldest first ⇩
Q. We had a customer request a job, defining it as "202 clear anodize". Is anyone familiar with this particular aluminum anodizing specification?
Mike Solvie1998
A. 202 Anodize refers to an AA Aluminum Association Spec
- 204 = 30 Min or equiv anodize
- 215 = 60 Min or equiv anodize
- 202 = Thickness to be specified
- Long Island City, New York
A. Alumilite 202 is an Alcoa designation for a 0.3 mil thick, clear anodic coating with a boiling water seal.
Chris Jurey, Past-President IHAALuke Engineering & Mfg. Co. Inc.
Wadsworth, Ohio

A. Back in the 50's & 60's ALCOA promoted a licensing system called Alumilite, and they originated the 2xx numbering system. The 204 and 215 which Mr. Kraft cites from the Aluminum Association pretty much follow the original Alumilite 204 and 215. According to an ALCOA reprint I have from 1965, the Alumilite 202 called for 0.3 mils of anodize with a boiling hot water seal.

Phil Johnson
- Madison Heights, Michigan
Q. We assemble watches and have encouraged a supplier making Aluminum cases for us to switch from 1100 alloy to 6061 to get a stronger part. They just reported that they have trouble clear anodizing the 6061; they say it goes yellow. Before I visit them, what should I be looking for as the cause? I understood 6061 should be very suitable for all kinds and colors of anodizing. Is contamination the most likely cause ?
Hamish Low- Hong Kong
1999
A. It is an industry wide problem that tables produced which state that certain alloys anodise OK do not distinguish or define what constitutes aesthetic acceptability, what is acceptable on an industrial machine tool may not be acceptable on a piece of jewelry.
The 'problem' you refer to is not at all unusual, indeed almost predictable. The yellowish hue you are seeing is almost certainly associated with the copper content in the alloy and not with anything that the anodiser is doing incorrectly. Aluminium alloys with copper in them are usually used because the copper makes them easier to machine, however, when anodised the copper atoms become incorporated in the anodic film and give a yellow hue to the finished article since they are not translucent in the way the aluminium oxide is. Generally the thicker the film the more noticeable the colour change as more copper atoms are incorporated. From a technical view-point the product is probably fine, (corrosion resistance, abrasion resistance etc) however with a product such as a watch I assume that there are aesthetic requirements as well and this is actually were your problem lies.
Possible ideas to help, ask your aluminium supplier to supply alloy at the low end of the copper content range, (allowable range for Cu is 0.15 to 0.4) and if the product allows anodise with a thinner film, but note that with a thinner film you will probably sacrifice technical performance for improved aesthetics!
Good luck!
Peter Hirst- UK
Multiple threads merged: please forgive chronology errors and repetition 🙂
Trying to match clear anodizing on 6061 and 6063
Q. We are trying to clear anodize flat stock 6061 to match angle 6063 -- the 6061 is always darker and I cannot get the 6063 to go darker without over-anodizing the contact points. We are racking them all on titanium racks. Any suggestions?
Teri Andrews- Albany, Oregon
2002
A. The most frequent tech service advice to a telephone or finishing.com question is "GET THE CURRENT ON THE PART". Rack the part so that it gets its current from an aluminum bar contact and then use titanium merely to spring hold the part against the aluminum -- get the current on the part. Your two alloys should come out with the same clear shade if properly deoxidized properly contacted, 72 °F, 15%/wt Sulfuric Acid, under 12 gm/L dissolved aluminum, and for the same anodizing time.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

A. Teri,
I believe you are battling the amount of Si thats in 6061, but not present in 6063. My only suggestions would be to either lower the current density when anodizing the 6061 (leads to lighter coatings), or, perhaps lower the temp when anodizing the 6063 (leads to darker coatings).

Marc Green
anodizer - Boise, Idaho
A. I should have done a little more research before I replied to this letter. While I was partially correct about the Si content in 6061 compared to 6063 (.4-.8 Si for 6061, and .2-.6 for 6063) the range for other alloying constituents ( most notably Cu, Cr, and Zinc) is also much higher for the 6061. All, of which, can lead to the color differences that you are seeing. Me thinks, that even if one tightly bolted into both alloys and put them through identical processing conditions for a type 2 class 1 process, that the color differences would still be evident (if I have some 6063 around the shop, I will run this test, photograph, and post the pictures). I used to use racks that had 6063 splines, and 6061 fingers, and after processing, the color difference was striking (although, I'm sure some current robbing was taking place).
Opinion! I feel the need to get on my soap box for a minute, so please indulge me. I wish that the aluminum manufactures would find a way to tighten up their processes, and give us a consistent, quality product, with each mill run they produce. In this day, where quality, and consistency, is of the utmost importance...this is an issue I wish would be addressed. Letter 14352 (by the way, Katrina, if you are reading this, I would suggest skipping or reducing the NaOH etch..this may reduce/eliminate the mottling you are seeing), along with many others, and my own personal experience, clearly indicates this need. I've come across differing tempers (hardnesses), differing colors, differing growth rates, mottling, grain structures -- you name it -- all out of the same alloy. And we, as anodizers, must deal with the end result, and try to explain it to our customers that its the aluminum that is perhaps causing the problems/inconsistencies, as opposed to our process. Which, a lot of times is very difficult -- after all, aluminum is just aluminum, right? WRONG! My company has dealt with the big manufacturers on this issue, and all that we get told, is that the Al is "in spec". It can be, at times, very frustrating. I suppose, if my specifications on a 2 mil hardcoat was anywhere from .0005" to .00035", my parts would always be "in spec" too ... but alas, I am held to a higher standard. I, for one, get a little tired of having to tweak my process to compensate for inconsistencies of different lots of aluminum for the parts that we manufacture and coat. The differences clearly show up with EIS, hardness, SEM/x-ray, and dielectric strength testing that I/we have performed...but yet the material is all "in spec".
Ok, I feel better now. Thanks for indulging me ... ya'll have a great week.

Marc Green
anodizer - Boise, Idaho
Should clear anodized finish be dull or shiny?
Q. I have two parts from two different vendors and the clear anodized finish is dull for one and shiny from the other. From what I understand the dull finish is typical of a clear anodized part; is this correct?
Bryan RolloManufacturing Technician - Smithfield, Utah, USA
2007
|
A. Assuming the alloys were identical, assuming the heat treatment was identical, assuming the starting finishes was were identical; some other differences could be caused by:  Robert H Probert Robert H Probert Technical Services Garner, North Carolina  A. It is dependent on many factors. I assume that the surface finish started out exactly the same. Cleaning times, temperatures, type of cleaners/desmut/etchants and concentrations will all affect the finish. James Watts- Navarre, Florida A. No. A dull finish usually indicates a dull surface prior to anodizing. Shininess does decrease with increasing anodizing thickness, especially for highly alloyed aluminum. A dull (matte) finish is often intentionally produced via etching or bead blasting in order to hide scratches and machining marks. The Aluminum Anodizers Council's Anodizing Reference Guide may be helpful in specifying mechanical and chemical pretreatments as well as the anodizing: http://www.anodizing.org/reference_guide.html - Goleta, California Rest in peace, Ken. Thank you for your hard work which the finishing world, and we at finishing.com, continue to benefit from. |
Q. I have seen this question raised several times on this forum (letters 19453, 33304), and the response seems to be a lack of coating thickness and/or poor sealing. We are having this problem now on all of our aluminum components. This is a new problem for us as we have had material coated for years. The problem is - it is not an issue we can direct to 1 coater or material type. We have machined parts, parts formed from sheets, and extrusions which are turning yellow/green. Finish doesn't seem to matter either - be it tumbled, bead blasted, brush finished, or no prep at all. There are several different platers being used, included parts purchased complete from overseas - (China).
It appears that the discoloration takes place once the parts are exposed to plant conditions. We have a clean environment, nothing out of the ordinary as far as atmospheric conditions. Lighting is your basic florescent fixtures and metal halides. We are stumped, and our platers are stumped. It appears to me that the lighting is causing the anodize to turn - has anyone heard of this before? We can have the parts stripped and replated, only to have them to turn color again. The yellowing can also be removed using a mild paint stripper - (just testing - probably not a solution..)
We really need some help here - we are trying to protect our interest here - our customer will surely reject our product if it is received or turns yellow at their facility.
Manufacturer / Assembly - Cleveland, Ohio, USA
January 26, 2012
A. 1. Is heat involved ? 2. On some incoming parts, perform an ASTM seal test, if they pass, then leave in the plant the normal time for the fading to happen, if they fail the seal test then you know why. Most job shops do not control their seal and do not perform seal test, so YOU do the seal test and determine whether sealed, then come back to us.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

Clear anodising - Appearance and color variation
Q. Hello,
I have many different parts in aluminum 6061-T6 and I want them to have the same light grey color.
I have in mind the iPad aluminum body finish.
I sandblast all the parts before clear anodising because I want to have a uniformity between the rough surfaces and the machined surfaces.
Presently I don't have the uniformity I want.
How can I achieve it?
Any help will be appreciated.
Best regards.
packaging - Montreal, Canada
September 14, 2015
A. Good day Terry.
See letter #18655 from David Hendrick regarding blasting and uniformity, or the lack of it.
There is very good info here at Finishing.com.
You just need to look for it.
Regards,
Lab Tech. - Whitby, Ont., Canada
A. First be sure the sand blasting is UNIFORM because anodizing magnifies ALL defects. Be sure the sand blast media is designated for ALUMINUM only and not loaded with some night shift man's iron gun parts. The racking on all parts must deliver the current evenly to all parts on the rack. The agitation must be uniform, air does not hit parts displacing solution, and the solution moves as much behind the part as in front of then part. Back up stream the time in the etch tank is always the same.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

Clear anodizing, how to get the best clarity
Q. I am anodizing on a small scale for a couple of years now, mostly black (glossy, satin and matte).
Small automotive interior parts milled/turned by myself (shift knobs mostly) from 6082, previously 5083 but with lesser results especially with black dying.
I've never had any pleasing results when trying to clear anodize brushed or polished aluminum, it comes out much more dull even when polishing the anodized layer again.
I had slightly better results this week on some 6060 but still the beauty of the fine brushed aluminum is completely lost after the process in my opinion.
I'm confident I've seen clear anodized aluminum that was much clearer then what I'm achieving but I can't seem to find much specific information about this.
Obviously there will always be more dullness afterwards, but what could I try more to achieve the most "clearest" results?
Current process when clear anodizing:
Parts are basically always anodized within 30 mins. after finishing (sanding/brushing).
- 10 min. degreaser bath at 60 °C
rinse
- 2min. deox bath at room temperature
rinse
15 min. anodize bath (15% sulfuric) 1 Amp/dm2
rinse
nitric acid rinse
rinse/rinse ionized water
chemical cold seal (1m/um)
Temperatures/durations as specified by chemical supplier.
Any help/advice would be greatly appreciated.
Small business owner, automotive interior parts. - Assen, Drenthe, The Netherlands
September 19, 2018
A. Clearer anodizing: Higher temp, higher concentration and lower current density. You did not say 15% what: weight or volume? Try 75 °F, 195 g/L acid, 10 ASF, and experiment plus/minus those levels.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

Natural anodizing comes out different tones
Q. The alloy is 6063-T5, after natural anodizing it was found to have different tone of color.
Why?

- Kapar Klang Malaysia
October 30, 2020
|
Q. Could it due to: - Kapar Klang Malaysia A. Probable varying electrical contact reliability.  Robert H Probert Robert H Probert Technical Services Garner, North Carolina  |
![]() Thank you for your advice,
Thank you for your advice,
our materials which were racked in the same bar for anodizing are from the same heat number of billets used.
Supposed to be of same chemical compositions.
What I propose to do is taking 2 bars of different tone material and rework, start from degreasing until sealing.
To see the color tone will be same or different.
BTW, our sealing here is cold sealing at room temperature 25 to 29 degree.
- Kapar Klang Malaysia
November 10, 2020
Extreme brown discoloration after clear anodizing
Q. Hi all,
First of all this forum helped me a lot understanding the anodizing process and I read a lot. I'd like to thank you all for that!
I'm currently project member in a frame building project where several aluminium parts are assembled onto each other.
One of these parts is a welded sub-assy frame of several parts from a thickness of 20 & 25 mm 6082 are welded.
After welding the frame is clear anodised (with matte pickling 40 µm). The images below shows the result: several plates were discolored, the other not.

We've been testing if the discoloring was removable by de-anodising/bursting and then anodise the product again, but the results were hardly better.
Specific questions:
a) Is this brown discoloration repairable?
b) What is the root cause of this discoloration?
Kind regards,
- The Netherlands
November 3, 2020
A. Hi Gertjan. The discoloration is under the anodizing and is not removable. Although some people consider 6082 to be "the European equivalent" of 6061 (or maybe 'European substitute' would be a better term), others believe the small additional amounts of alloying ingredients which offer its higher strength make it less suitable for aesthetic anodizing (you can search the site for 'anodize 6082 aluminum' to see the 2nd and 3rd opinions on this subject).
Regardless, it is obvious that the composition or temper of the 'yellow/gold/brown' pieces is different than the clearer pieces and it has reacted differently to some step in the process. I do not think it is possible to resolve this problem without assuring that all the pieces are the same composition and temper. I would start by requesting certifications for the materials which turned brown; they might not even be a 6xxx aluminum :-(
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
? Ted, correct me if my understanding is incorrect.
The word "discoloration" is something that of colour changing, not that parts coming out with different colour tone.
- Singapore
November 5, 2020
![]() Hello John.
Lim and Gertjan provided pics of their problems, so I don't think that semantics is critical in their cases. Lim called his problem 'different tone', and Gertjan called his problem 'discoloration', and I didn't/don't take issue with either person's use of their wording ... but I'm not the arbiter, and there perhaps could be cases where the precise meaning is critical :-)
Hello John.
Lim and Gertjan provided pics of their problems, so I don't think that semantics is critical in their cases. Lim called his problem 'different tone', and Gertjan called his problem 'discoloration', and I didn't/don't take issue with either person's use of their wording ... but I'm not the arbiter, and there perhaps could be cases where the precise meaning is critical :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi John,
Thanks for your quick response, we've asked the material supplier for the certificates. Those are within the spec.
Further searching on this forum did unfortunately not result in any similar issues...
If anyone has an idea, please let us know. Thanks in advance!
- The Netherlands
November 5, 2020
A. From the picture you attached, the brown colour area is the recessed area? how do you load the part? I mean you may take measures to avoid air pocket in the anodizing bath, but probably neglect air pocket problem in other tanks, especially the sealing tanks.
tell me whether another side of brown colour is in different colour tone?
- Singapore
A. I respectfully disagree with John that an air pocket could have caused this problem. The welding on the brown pieces is not brown, but even the edge of the brown pieces are brown. I'm confident that there is something different or wrong about the composition or temper of the plates that came out brown.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Lim, Gertjan
I am going to second Marc Green's 2002 post.
The tolerances for a material (aluminum) to be in specification are very wide. In comparison, the tolerances that most anodizers hold is very tight in comparison.
Intel drove down their "Copy Exact" program in response to variations in the performance of their 'tools', much of which was due to variation in the materials used. Suppliers of key components (used in the 'tools' Intel uses to manufacture semiconductors) were required to purchase a mill-run (~80,000 pounds) of aluminum to ensure that variations in these key components was not material related.
Prior to implementation of the program, we could identify the different materials used in a single lot or run of product prior to anodizing, even though the manufacturer / machinist claimed they were the same material. After anodize, there was a pronounced difference in appearance and a bi-modal histogram of the thickness.
As the anodize is formed of the aluminum, different heat lots of the same alloy can produce different appearances. Heat lots will have different chemical composition and temper which can affect the appearance of the anodize. Lim's and Gertjan's photos are characteristic of variations in the material.
Lim; consider taking a sample length from each of the two different 'colored' extrusions and immersing them for an extended time, both in NaOH and HNO3; the HNO3 immersion might be hours not minutes. If the materials are the same, they both will appear the same after exposure. If not, the difference in the material may be cause for the difference in the appearance of the anodize. The results won't fix the problem but it may tell you where it is.
Also consider measuring the thickness to see if there is any correlation. If you are anodizing to 5-10 µm, the difference will be very subtle if you do not sample enough.
- Green Mountain Falls, Colorado
Q. Hi all,
thanks for responding!
@John, both sides show brown spots although not always., and the intensity of discoloration is different.
@Ted, the material is also in our opinion what our most suspicious parameter is. No other process changes have been made since the last production run. We'll dive in to this even more than regular certificates. Do you think the intensity of pickling/anodising creates a better/more worse effect?
@Willie, useful info! thanks. If we're going to test if there is correlation between layer thickness and color we'll let you know. Have you experienced any correlation between layer thickness and color before?
gr Gertjan
- The Netherlands
November 9, 2020
A. Hi again Gertjan. In the USA we usually call that step "desmut" or "de-oxidize" rather than "pickle".
But in brief, as you surely know but we'll say for the benefit of readers, aluminum alloys are not pure aluminum; they are alloys of aluminum and other materials like silicon, copper, magnesium, and zinc. In the etch step the aluminum dissolves but some of those other materials are left behind and become concentrated on the surface. One of the purposes of the desmut is to try to dissolve away those contaminating materials; so you must choose a desmut solution and operating conditions that work best. The discoloration caused by those alloying materials is also certainly proportional to the anodizing thickness, which is why hard anodizing is usually gray to charcoal in color rather than clear.
So the answer is yes, you can hopefully minimize the tonal differences by minimal etching, optimal desmutting, and minimal anodizing thickness. But when even the welding comes out the right color, the process is probably pretty good, and the material that came out brown pretty bad :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Ted, we have every reason to suspect the material composition although Gertjan confirmed that there is no problem with the material.
Ted, we have every reason to suspect the material composition although Gertjan confirmed that there is no problem with the material.
I know that sealing process will shift the anodized colour tone to the lighter side, I don't know whether Gertjan has ever monitored the colour tone of parts just taken out of the anodizing bath.
Actually, if there is the material issue, in this case during pre-treatment (after etching) experienced worker should know already.
- Singapore
![]() Hi Both,
Hi Both,
I think we have lot to sort out and test with the material and process.
For now your info helped us thinking in the right way I guess. Thanks, and if there is anything new or solved we let you know here!
Greetings Gertjan
- The Netherlands
November 11, 2020
A. Gertjan
With respect to color versus thickness, for any given alloy, a thicker coating will have more 'color' for any given set of parameters. Parameters can be altered to lighten or darken the anodize for any given thickness. For any given thickness and associated parameters, alloy color can vary. For some (7xxx), the difference is pronounced. The purer the alloy, the less change per unit of thickness.
- Green Mountain Falls, Colorado
A. Hi Gertjan,
Let me know what is your desmutting bath's (neutralization) composition?
Is it only nitric acid based or sulfuric acid + oxidizing agent based?
According to your answer, I would advise how you can make a test.
- TURKEY Sakarya
![]() Hi Willie,
Hi Willie,
thanks for the overview of thickness/material influence on different types of alloys.
@allaattin,
Don't know the details yet. The next production run (not sure when) will be tested more specifically. Then I will have more info.
- The Netherlands
November 19, 2020
Q. Hello,
we have been having this "brown" discoloration issue with the material (6082) bought in last 6-8 months also, and one suspicion is I have is about salts used in precipitation hardening of the affected batches of extrusions.
What I've observed about this issue is that the "defect" is not uniform, meaning - the extrusion is cut into segments, then machined, and after anodizing (when the discoloration shows) I can line up the pieces and actually see graduation of parts that are darker, then consequent pieces become lighter to next pieces again getting darker and so on, this seems to be recurring effect throughout the batch of material.
The most severe parts affected by this (darkest discoloration) seem to exhibit sort of "runs", like the part would have something sweating out of it during anodizing and running down creating streaks - and this isn't a racking issue or rinsing, that much is certain, because it may affect a single part in a middle portion of the rack, parts above, next to or below are perfectly fine, not a loss of contact issue either, I monitor voltage and current and the parts are few enough and large in the lot that any loss of contact would show there.
So my suspicion is about precipitation hardening salts used by the manufacturer of the extrusion, I'm going to send a few parts to be XRF examined to hopefully see the "offending" element, but I have hard time finding information regarding what kind of salts are used in this process, I also suspect increased amount of silicone, but from previous experience it simply makes the affected parts grayer with no brown tint to it.
Hopefully others will chime in on this, it is becoming a rather big issue lately.
- Riga, Latvia
December 11, 2020
A. Hi Janis,
Have a look through your records and see if this is unique to one particular heat lot of material, or a recurring issue across multiple lots; also if material from one mill, regardless of heat lot, is more prone to this defect.
Though I've never seen the 'sweating' effect you describe, I've certainly battled enough horribly striped or swirly patterned components- made of everything from plates, bars, and rods, to custom extruded shapes- with obvious material defects that can be lined back up like a puzzle after anodizing.
Hopefully someone with a good explanation of WHY the dark streaks appear; whether it's a region of a higher concentration of one of the alloying constituents, or a problem with non-uniform cooling of the initial ingot, will chime in and explain it! I'm no metallurgist, but you should talk to your material supplier and inform them of the issue. This is a material problem straight from the mill.
If finding a new material supplier, or even rejecting any more shipments of that particular heat lot, is practical; I'd do that.

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Q. The "light" looking part and the "brown" looking part were anodized together on the same rack, they are matched sets, both parts in the set are very similar in surface area, but stock is different size (one part is thicker, both should be 6082 T6), nothing special about anodizing: degreasing, rinse, spray rinse, etch (40 gr/l caustic soda ⇦liquid caustic soda in bulk on Amazon [affil link] with additive @55~60 °C for 30 seconds), rinse, deox (sulfuric/nitric), rinse, spray rinse, anodize (sulfuric with high purity RO/DI water, 1.14 density, no additives, ~21 °C 1A/sq decimeter, minimal dissolved aluminum, titanium racks, current is achieved at 12,5V at the beginning which then raises to about 13,5 at 45 min mark) for 45 min which nets approx. 12 micron thickness oxide, hot nickel acetate seal - brown discoloration is not observed after etching ("zebra" stripes do show after etching) and before/after deox, but is present after anodizing.
We've used this same process for few years now with very repeatable results; this "brown" issue is new, and not all parts are affected. Suspected bad batch of material, but material analysis show little to no difference in both parts, and quite a few differences from the 6082 spec,. I asked to test the part as anodized with no surface preparation to hopefully capture the difference in oxide (if that "difference" hasn't dissolved into electrolyte), test was done with Optical Emission Spectrometer (PMI Master Pro), but I struggle to interpret the results, I'd welcome any comments.
Pictures A and B are stills from a video, hence a bit of a lack in quality
Picture A: shows the set of parts held together (parting line between the parts is about 1/3 from the top of picture, you'll see it much better in picture B) as supplied by the machining company. Notice the bottom part having a slight yellowish hue; it isn't noticeable at any angle in sunlight, but below LED overhead lights it can be noticed at certain angles.

Picture B: shows the worst case after anodizing (as described above). After seeing this result I omitted etching altogether and the result was slightly better, a little less "browning" but worst parts still had the "zebra" stripes along extrusion direction (red line) - it is a material/grain issue - very noticeable when flats on the part are compared to curves.
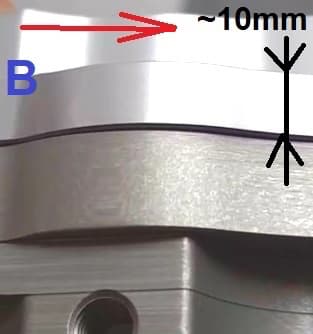
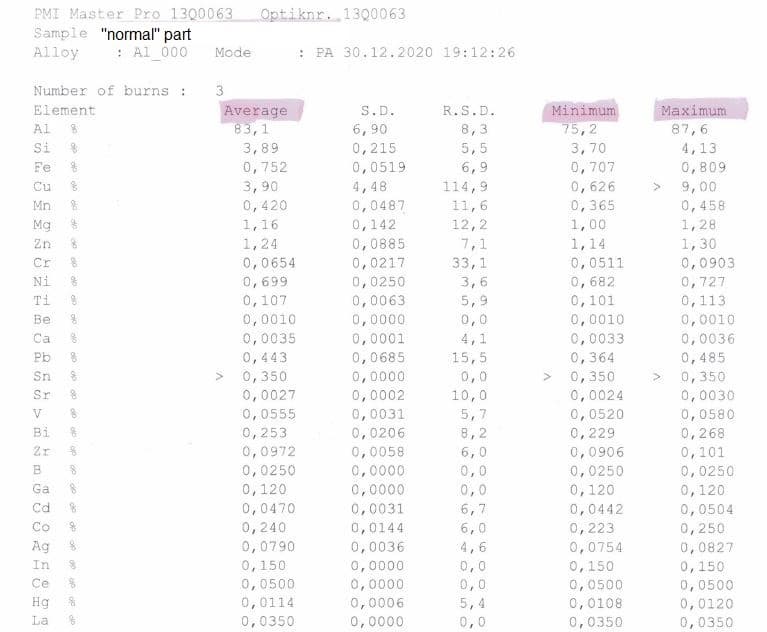
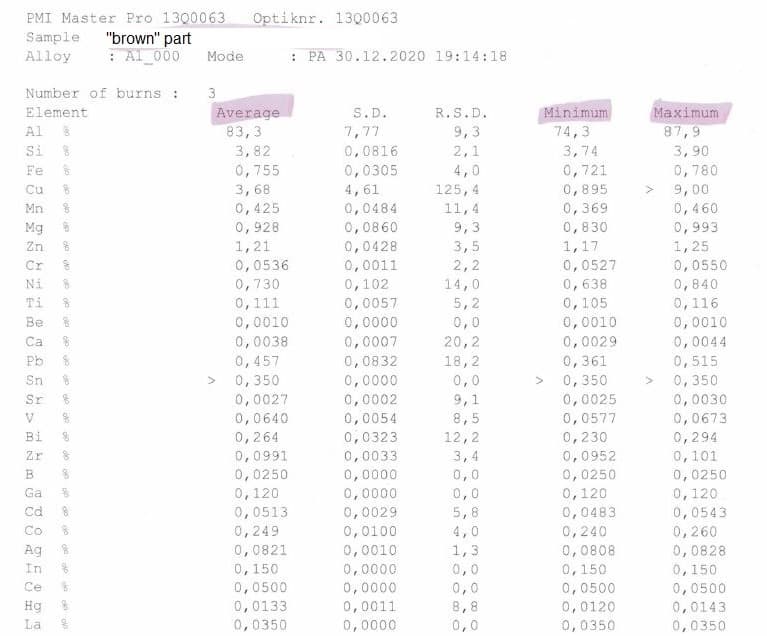
Thank you very much for all the work you're doing with the site.
Janis Ziemelis- Riga, Latvia
January 6, 2021
A. Hi Janis. Is there any chance (considering the different thickness/shape) that the two components were machined on different equipment and that one of the machines used different machining fluid, or overheated it, such that it does not get fully removed in your caustic cleaner? Could you pre-scrub a few parts with a pumice solution to learn anything?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Two parts seen in the pictures are machined together only in the last operation (there is a feature on them that requires both parts to be assembled, fixtured and machined), and that is where the yellowing occurred on one of the parts and not the other, it wouldn't wipe off with a finger, but they said it did with alcohol, I didn't test that at the time, but it seemed to me that it did disappear after degreasing (Anodal BFO-2 degreaser, 5min@55C), but since it was barely noticeable anyway, I figured that the water film on the part may have hidden it.
Regarding the test results, they can be discarded, initially I tasked the testing company to use XRF method to make a comparison between the 2 anodized parts, but later they called over the phone and said they'll perform OES instead, at that moment I didn't know the details and agreed, but it was very obvious to me why it failed to produce usable results when I asked to see the instrument, the business end is a metal contact that forms one part of the electric circuit to generate the plasma, and it failed to make good electric contact with the part (obviously, because it was anodized...), there were few spots where some spark erosion has happened, signs of it on the instrument as well, which I'm guessing contributed to the confusing testing result. To be fair they did say they need to sand off to bare surface, but I told them to try the test anyway, I wasn't aware of the details of how it worked then.
I'm still not sure why they asked to switch the testing method, but right now we'll simply prepare new samples to be tested with the same OES method, not anodized, and in the meantime look for someone else to do the XRF analysis on the anodized parts, hopefully we'll somewhere with this, I'll update as I learn something new.
- Riga, Latvia
January 6, 2021
A. Hi Janis,
I suspect your anodizing process parameters. Firstly, why don't you increase caustic soda ⇦liquid caustic soda in bulk on
Amazon [affil link]
amount until 65-70 gr/lt? Up to me, 40 gr/lt is low amount for keeping balance of dissolved aluminium/caustic soda. Also you can increase current density of anodizing bath from 1 amp/dm2 to 1,5-2 amp/dm2. If I were you I would test both, changing and surveying the result. Your problems which you mentioned "zebra effect" and "yellowish-browning" might be solved by this process changing. Also, as last advice, you would skip etching process as test and you can survey non-etched anodized parts for both defect aspect. At least you would understand where is the source of problem depending on results.
- TURKEY Sakarya
A. Janis
I agree with Rachel that you might want to look into the aluminum components coming from 2 different heat lots.
The "B" photo suggests (strongly) that they are different heat lots. The runs/zebra stripes mentioned is the grain structure. Most pronounced at the inside radius on the right where more of a single grain structure is exposed / elongated.
Both etching and anodizing will expose / highlight the grain. Reducing the etch time will help, but not eliminate it.
You might be able to utilize an eddy current thickness tester to identify the different lots prior to anodize. That would go a long ways towards convincing the manufacturer that root cause is the material used to make the parts.
The procedure will work best if you have a sampling of the same part number. Take one part and standardize / normalize the thickness tester on a specific surface for ZERO / base and for a 2 or 3-mil shim ( 5-mil maximum). All subsequent testing should be done on the same designated surface; part / grain orientation will affect the results.
Once standardized, measure ZERO and the shim value (used for standardizing) on each of the parts. The parts made from a heat lot other than that used for the standardization part may exhibit a shift in the mean values obtained (either for ZERO or the shim value or both). You might need to run the experiment using a different surface with respect to the grain orientation to get the best results.
I've suspected the quench rate and / or temperature as the variable that is out of control causing this issue.
Good luck
- Green Mountain Falls, Colorado
A. There is no question to me that the discoloration is from the Alloy used. It would not be the first time a metal supplier sent something without making sure that the alloy is the correct one.Different alloys of aluminum anodize with a different color Hue. AAC has a chart that defines the hue of the anodizing after it has been done. Some are not pronounced but can still be seen when next to another alloy.If you email me I can send the info to you or you can visit "the aluminum anodizing council"

Drew Nosti, CEF
Anodize USA
Ladson, South Carolina

A. Hi
Color differences can often be observed in alloy 6082. Partly on the composition, but also often on the structure.
The micro structure consists of primary and secondary phases. The secondary phases are mainly formed during the heat treatment. Different cooling conditions or different heat treatment processes can result in micro structural differences. Many secondary phases can cause the anodized layer to turn gray or brown. Differences can sometimes be noticed after etching (different coverings).
Structural differences can be determined by examining the microstructure on the metallographic section on the light microscope (special etching required).
Simple method: measurement of the electrical conductance, many secondary precipitations can cause a higher conductance. Differences in the conductance indicate structural differences.
Was the electrical conductance measured (MS / m,% IACS)?
- Hallau, Switzerland
A. Aluminum Finishing
All alloys of aluminum have different constituents. Some are high in copper others high in Zinc. It really depends on the alloy and what lot or pour it comes from. Now since the oxide is formed from the base aluminum the metals in the alloy will have some affect on the tone of the oxide created. This creates a problem when it comes to anodizing parts and trying to match color. The fact is that even if the correct alloy is used it is sometimes impossible to tell if the pour is the same. Aluminum is poured in large batches and therefore it becomes impossible to be sure that all the parts you intend to anodize are from the same pour. Distributors of aluminum have no need or requirement to insure all the parts are being made are from the same lot/pour, they just supply the aluminum based of size and maybe alloy.
There remains some ability to compensate based of pretreatment /post treatment and dyeing if needed. But it is important to remember that the constituents of the oxide will have a bearing on the final finish when anodizing. Compensating for differences in the lot usually is difficult because it shows up after the parts are done and they do not match what you expected. Here are some ways to help correct this problem they may or may not work depending on the problem:
1- Limit or eliminate the etch prior to de-ox.
2- Increase the De-ox time
3- Add a fluoride in the de-ox.
4- Test random samples of the batch before the final run and adjust time/process.
5- Drink heavily (it will not solve the problem but you will not care)

Drew Nosti, CEF
Anodize USA
Ladson, South Carolina

Q. 6061 anodizes like its 6063.
I've been anodizing 6061-T6 for about 9 yrs now on a hobby level and have experimented with 6063 on occasion. At one point I received a batch of parts from a supplier they claimed were 6061, and during anodizing, they did not turn the typical golden champagne color I'm used to. As a matter of fact, they did not change color at all, instead retaining their silvery finish post anodize. The process wasn't to blame as I would anodize these parts with other material from a different batch, and those parts would turn the typical champagne color.
I had one of the parts sent out for material analysis, and all component percentages came right in line with 6061, as shown below:
Silicon------------ 0.67
Iron---------------- 0.4
Copper----------- 0.31
Manganese------ 0.08
Magnesium------ 1.0
Chromium------- 0.09
Nickel------------- 0.02
Zinc---------------- 0.05
Titanium---------- 0.03
Lead--------------- 0.01
Tin----------------- <0.01
Beryllium--------- <0.01
Vanadium-------- 0.01
Zirconium-------- <0.01
Aluminum-------- Rem.
Check out the below pics. These parts were all anodized on the same rack and thus under the same conditions. The red box indicates my typical 6061 that turns a deep champagne color after anodizing. The blue box indicates the "magic" batch. No color change whatsoever from pre to post anodizing. If I dried it off, you'd think it was fresh off the CNC machine. The purple box also indicates the magic batch, but this part must have been machined from a different lot of material. It turned a very slight shade of champagne which may be difficult to see in the pics.


The final picture shows both parts from the magic batch. The one on the left was in the blue box in the previous pics (no color change pre to post ano). The one on the right was in the purple box (slight champagne color). Notice the more vibrant coloring and shine on the part on the left.

Any thoughts?
Dave Demaree- Dade City Florida
April 7, 2021
A. Dave
Perhaps the 'magic' batch has a temper of T6511. Maybe ask whoever manufactured the components for the material certs, or if the raw material / bar stock is still labeled from the mill.
- Green Mountain Falls, Colorado
Q. Thanks for the reply.
I was never able to get a cert from the machine shop who made the magic batch (China). I've since moved all production to the USA.
Currently I have several batches made from the temper you mentioned and they all anodize the same (champagne color post anodize).
- Dade City Florida
April 16, 2021
Q. Dave here from Michigan. Trying to locate what is causing this vertical brown arc on some of my parts. Using the correct AMPs, racks are stripped completely, aluminum content below 10 and Sulfuric Acid content @ 245. I have 4 anodizing tanks and this issue is not isolated to any particular tank.

Dave
- Wyoming, Michigan
January 15, 2022
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
|
|
A. It seems like "burning" when I look carefully on pic. Normally, if current density up to 25 asf and more then we can expect this defect, but you mention all parameters is ok. So I will suggest you differently and maybe it will be irrelevant to you: I encountered a similar defect a couple of months and the problem was solved by installing a new degreasing bath. I emptied degreasing bath and installed new bath. It's worth a try if you don't find source of problem.. Anodizing Supervisor - sakarya, Turkey A. Hi Dave supervisor - Cicero, Illinois Saporito Finishing Co.  |
Q, A, or Comment on THIS thread -or- Start a NEW Thread
