
Home of the world famous 'finishing.com HOTLINE' since 1989
-----
Plating Thickness vs. Amps/square foot?
Q. Does anyone here have a guide or Excel spreadsheet that helps with thickness if you put in the amps per square foot, quantity, and thickness required. I'm looking to make one if not.
Does anyone have the formulas for sulfamate nickel and pure tin.
Plating shop - Anderson, South Carolina
October 13, 2008
A. Hi, Mark. The thickness guide is actually Faraday's Law of Electrolysis, which says that 96,485 ampere-seconds will deposit 1 gram equivalent weight of a metal. This is based on the rules of chemistry, and always applies if the plating process operates at 100% efficiency (i.e., if no electricity is wasted generating hydrogen instead of a metal deposit).
Near the back of the Metal Finishing Guidebook you will find a table which takes into account Faraday's Law, plus the densities, oxidation states, and atomic weights of the various plateable metals, resulting in a a chart of thickness deposited per amp-hour for each metal. I think this is what you are looking for. The nickel sulfamate is straight forward, but remember that tin is in a different oxidation state in stannous sulfate solutions vs. alkaline stannate solutions, so the thickness per ampere-hour will depend on which plating solution you are using.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Mark,
The amount of metal deposited is governed by Faraday's Laws of electrodeposition. One of these states that 1 Faraday will electrodeposit
1 gram-equivalent of metal. A Faraday is (approximately) 96,485 A-secs or coulombs. The equivalent weight of a metal is its atomic weight divided by the oxidation state of its ionic form (for nickel this is usually 2, but tin can be 2 or 4 depending on whether it is acid or alkali tin). This, of course, assumes 100% efficiency, which is not always the case. I suggest you refer to a good electroplating text book (e.g., The Canning Textbook Surface Finishing Technology), where all the relevant information can be found.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
October 15, 2008
Q. Good Day,
We do strip-to-strip electroplating. We have a target plating thickness which needs to be achieved. We have the ASD (amps/square decimeter) for the coating material, the target thickness and the surface area part to be plated.
We need to know how do we calculate the theoretical immersion time and the required current which needs to be applied in order to achieve the thickness.
Also are there any difference in the calculation for electroplating with soluble anodes vs the electroplating with insoluble anodes.
Thanks in advance for your help.
- KL Malaysia
August 9, 2012
A. Hi, Rajesh.
We appended your inquiry to a prior thread which explains the relationship between plating thickness vs. the applied amp-hours per unit area. This then tells you how much the product of the plating time x the current density must be to achieve the desired thickness. But knowing the product of the amperage x the time isn't quite the whole answer because you're not really free to pick an arbitrary amperage and then calculate the plating time, because not all amperages are workable. If the ASD/ASF is too high, for example, the plating will burn instead of deposit correctly. At the stage of science we have reached, ASD is still pretty much considered an empirical number rather than a calculated one.
Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Plating Parameters calculations
Q. Hello all,
I'm new to plating and need to calculate plating parameters based on target plating thickness and the surface area of the part to be plated.
I need to know how to calculate the plating time and the required current which needs to be applied in order to achieve the thickness.
I'm plating Au, Ag, Ni & Cu. Right now I'm trying to calculate for plating 13 microns of Au on 2,551,230,254 sq. microns of plate-able area.
I'm not sure if I could pick my current at 50 mA and then calculate the time and if so what is the formula to achieve that?
Thank you for your help.
semiconductors - Chestnut Hill, Massachusetts USA
March 24, 2020
A. Hi Khody. Although there is a lot of science in electroplating, there are still some things that we know only empirically ... and one of these is the maximum acceptable current density that can be utilized without generating "burning". Most people want to plate as fast as they can, so they want to use as high a current density as they can, but can't allow burning.
Although deep technical insights are beyond my pay grade, basically what happens is that ions need to traverse the tank from the anode to the cathode. In doing so, they hit a bottleneck at the cathode boundary layer where there is no solution movement, and where the magic of diffusing through the boundary layer and being converted from ion to atom occurs. If you apply too much current, there will not be enough metal ions available to accept all the electrons at the cathode surface, and those electrons will split water into H2^ and OH- instead. Or the electrons will grab the available metal ions so hot & heavy that poorly adherent powdery metal forms instead of the metal growing on the cathode in its orderly crystal structure.
So 'allowable current density' is an empirically determined thing and it depends quite a bit on the mechanical arrangement. In semiconductor work maybe you are doing reel-to-reel plating of strips? If so, you may be aiming a strong stream of plating solution at the strip, which thins the boundary layer and allows faster plating. In the more general case of rack or barrel plating this approach is less feasible so maximum allowable current density is much lower.
Once you know the maximum allowable current density for your equipment and process solution, the rest is the pretty routine math of applying Faraday's Law and adjusting for the efficiency of the solution (another empirical value).
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 2020
|
Q. Hi Ted, semiconductors - Chestnut Hill March 25, 2020 A. Hi Khody  Geoff Smith Hampshire, England March 25, 2020 |
(affil links)

free pdf is currently available from academia.edu
![]() Thanks, Geoff.
Thanks, Geoff.
Hi again Khody. The chart that you are looking for can usually be found by searching the web for "Electrochemical Equivalents". One place you can currently find it is page 866 of the 2012-2013 Metal Finishing Guidebook, which is available for downloading from academia.edu.
Basically, if you pick a thickness of plating you want, and you know the area that you are plating, that gives you the volume of metal you must deposit. And if you know the density of that metal, you know how much weight you must deposit. And if you look up and know the atomic weight of the metal, you know how many gram atomic weights you must deposit. And if you know the oxidation/valence state of that metal, you can multiply the number of gram atomic weights by the valence and get the number of Faradays you need. And if you know the number of Faradays, you can multiply by 96,485 to know how many ampere-seconds you must apply at 100% efficiency. And if you divide by the efficiency you end up with how many ampere-seconds you need :-)
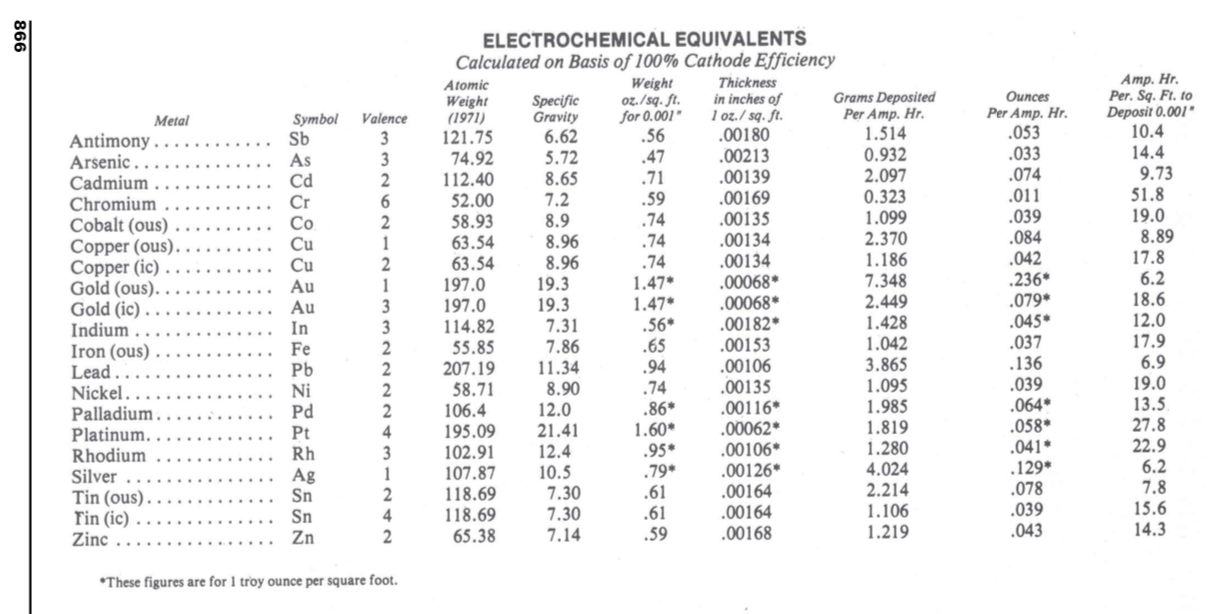
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Hi Geoff and Ted:
Hi Geoff and Ted:
Thanks again for your help and replies, I will need much more learning and practice!
Khody
- Chestnut Hill, Massachusetts USA
March 29, 2020
Q. Hi.
Good day to all
We are electroplating hard chrome for (solid) round bars with a maximum thickness of 35 microns.
The thing is, according to our report, 0.8 microns / minute @ 50ASD should be added. But currently it only deposits 0.5 to 0.7 microns thickness / minute with a single ASD (50ASD)
Looking for a solution to overcome the problem.
- India
June 20, 2020
A. Hi Potlada.
50 ASD is 464.5 ASF. So, per the chart I offered, if chrome deposited at 100% efficiency, it would deposit 464.5/51.8 x 0.001", or 0.00897" in an hour ... or 0.0001495" in a minute ... or 3.80 microns in a minute.
Therefore 0.8/3.8 would be 21% efficiency, which sounds like too much to expect unless you are using a HEEF plating bath.
And 0.7/3.8 is 18.4%, which strikes me as quite respectable.
So I'd probably overcome the problem with a dab of whiteout on your report :-)
Good luck, and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
June 2020
No dead threads!
Your Q, A, or Comment puts this thread on The Finishing.com HOTLINE.