-----
Is this ore/rock Platinum, Palladium & Rhodium? A meteorite?
Quickstart:
Please be alert to scams! Rocks are pretty much the most common thing on earth, and rhodium is the rarest metal on earth (35X rarer than gold!), so the odds of stumbling upon a rock with a measurable amount are astronomical.
Still, comments are solicited and offered on a variety of photographs and test results. Readers can learn at least a little about how to proceed by studying the comments.
There are a few youtube videos on the subject but they involve the use of very dangerous acids like aqua regia, which is only for trained chemists with hazardous materials qualifications.
Q. I have a mineral that I think is 95% rhodium. It is very heavy. It does nothing when heated to high temperatures. It is non-magnetic. ⇐ answer?
Shak sujonWarkar - Kuala Lumpur Petaling jaya
January 23, 2025
Q. What kind of stone this is? ⇐ answer?




Melissa Garrett
Hobbyist - Dallas Texas
September 2, 2025
A. Hi Melissa.
Times have changed and there are now several free smartphone apps for a first cut at rock and stone identification. Please see ecocation.org/free-rock-identification-apps/ for a review of 5 of them.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇩ Related postings, oldest first ⇩
Q. Hi, I need help with identifying a rock I have found. I'm no rock expert by any means, just a weekend metal detector guy. I've found a sample that fits the description of all 3 and my local jewelers say they have no idea except it ain't gold or silver or aluminum. Can't seem to find any good pics online or anyone who can tell me how to test this thing. Would be glad to send some pics if needed. The best description I can give is that it is flat, silvery dark grey in color. & very heavy, it weighs in at 3.185 ounces.
Any help would be appreciated.
hobbyist - Riegelwood, North Carolina
2007
A. Charles,
Why don't you go to a local Assayer ... presuming/hoping that you have one in your neck of the woods.
An 'assayer' is nothing more than a mining chemist ... but would have/should have all the testing equipment to determine what you sample might be.
... and if it's solid Platinum, please contact me IMMEDIATELY! I'd like to get some shares!

Freeman Newton [dec.]
R.I.P. old friend (It is our sad duty to
advise that Freeman passed away 4/21/12)
A. Look for a metallurgy lab around, they will analyze it for a decent price.
Guillermo MarrufoMonterrey, NL, Mexico
A. It will cost you a good bit of money to have it analyzed by spectroscopic methods.
While anything is possible, I doubt if you found a "chunk" of any of the precious metals that you mentioned. If it is, I would be looking for more, before someone else finds it.
My guess is nickel or cobalt. Put a drop on pure nitric acid on the brightest spot. If it turns green it might be nickel. If it turns a blue or purple, it probably is cobalt.
You might put the whole rock or part of the rock in swimming pool acid to see what happens. Some kinds of rock dissolve and others do not. Some metals will dissolve and others will not. Save the solids in a vial and the acid in a plastic container for possible later analyses.
- Navarre, Florida
A. Avoir du pois ounces?
Probably all three or else none if it's truly natural. Description of native metallic platinum:
"Platinum is usually alloyed with several percent Fe and with smaller amounts of Ir, Os, Rh, Pd; also, Cu, Au, Ni...[Specific Gravity] 21.45 when pure, 14-19 when native. Malleable and Ductile. Color steel-gray, with bright luster. Magnetic when rich in iron."
-- Manual of Mineralogy, 21st Edn., page 340, ISBN 0-471-57452-X. ⇨
1) Hang by a string and check with a strong magnet ⇨
for attraction. A very weak attraction is good.
2) Determine the specific gravity. One method is the Jolly balance (ibid., p. 257): Hang the specimen by a fine wire from a spring balance
⇦ on
eBay
or
Amazon [affil link] . Weigh in air and then submersed in room temperature water. The difference gives the weight of water displaced and hence the specimen volume. Higher weight is better.
3) Heat with an ordinary propane burner. Nothing should happen (oxygen is also required for melting). Poisonous fumes or burning are not-so-good. Possibly, a jeweler friend familiar with casting can try melting in a ceramic crucible. The higher the melting point the better; Pt & Rh have considerably higher melting points than the cheaper Pd, but expect alloying to lower the m.p. by as much as 200 °C.
4) Have a laboratory analyze the composition using an SEM equipped with EDS analyzer (EDAX) [SEM-EDAX on
eBay]
. Ask for at least 5 points on each of 2 sides, plus photographs of elemental distributions, and a signature on the report. Nice to know; necessary for selling.
The wet chemistry & pyrometallurgy of assaying and refining the platinum group metals involves strong acids, including aqua regia, and thermal decompositions giving off toxic gases such as SO3. Just a hint: Nitric acid will dissolve only Pd and the less noble impurities, aqua regia will dissolve both Pd & Pt, while the residue after aqua regia leaching can be fused in molten anhydrous sodium bisulphate ca. 315 °C to form rhodium sulphate. Solubilize, filter, precipitate, dissolve, precipitate...thermal decompose. Several such purifications give finely divided metal powders which can then be melted by electron beam in vacuum. So, skip the chemistry -- you might take it apart, but not be able to put it back together (in separate parts).
High hardness is expected, multiple alloying components may raise the hardness by 3x. Can't say much except soft is bad (unless have a nearly pure metal), maybe a sign of lead, silver or mercury.
5) With results 1-4, you can fill out a request to quote from a reputable company to whom you would mail the material, and receive payment after the assaying and refining costs are subtracted. Use an established company listed on a stock exchange (NY, London or Toronto).
Good luck.
- Goleta, California
Rest in peace, Ken. Thank you for your hard work which the finishing world, and we at finishing.com, continue to benefit from.
2007
A. I have been studying a rich platinum gold ore deposit and have broken open a ton of it. I can recognize it if it is rich enough. Platinum occurs with high iron content and sometimes produces black platinum crystals and nuggets; I have a bunch of them.
David Briggsmountaineer - Sonoita Arizona
May 23, 2023
Q. Hello, Can anyone tell me where to refine PGM in Thailand ? I have 1kg of raw platinum ore with 48 percent platinum and other platinum group metals in this ore , can I know how much amount of rhodium and iridium are present in this ore?
⇐ answer?
With regards,
- Thailand, Bangkok
May 16, 2013
Q. I have rocks that creates its own sulfur. What kind of rocks do this? I would like to know what kind of rocks create its own sulfur. I also believe it to hold platinum. Does this sound familiar? ⇐ answer?
Danielle Moorestudent - USA
February 25, 2015
Have I found Platinum?
Q. I found this piece of molten metal sticking out of a riverbed behind my house at first I thought it was lead that somebody had spilled out of the crucible but when I brought it home I put a propane torch to it on high and it took almost 5 minutes to even get it hot it never turned red a magnet does not stick to it it's very dense and very hard it does not throw Sparks on a grinder and has the patina like silver let me know if you have any helpful info. Thank you.
Billy GilmerPlumber and artist - HUACHUCA CITY ARIZONA USA
November 21, 2016
A. Hi Billy. The easiest first step is to determine its density or specific gravity. Weigh it, then immerse it in water and weigh the water it displaces. The specific gravity is the weight of the item divided by the weight of the water it displaces. Alternately, Ken Vlach offered a less messy and faster method: weigh on a spring balance
⇦ on
eBay
or
Amazon [affil link] in air and then in water. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
A. Hi Billy
Platinum melts at 1770 °C - way hotter than any other common metal. You might just be able to melt a small piece with oxy/propane but oxy/hydrogen or an induction furnace is more usual.
My second point is that platinum is very high value. People don't just lose pieces in a river.
Natural alluvial platinum occurs in Colombia and the Ural mountains of Russia. Finding a piece in Arizona seems unlikely.

Geoff Smith
Hampshire, England
Q. I did the specific gravity test and got 6.32 so it's looking like I have a piece of melted crap unless somebody knows otherwise because I couldn't find those numbers on the chart I downloaded.
Billy Gilmer [returning]Plumber and artist - HUACHUCA CITY ARIZONA USA
A. Hi. It's not platinum because that would be more than three times heavier. It's quite unlikely, but perhaps not impossible, that it's a silver alloy -- which you could test with a relatively inexpensive kit. It could be tin. But if I was forced to bet on a guess, mine would be that it's discarded zinc pot metal, which would weigh just about that if it had some aluminum in it like it usually does. That stuff is pretty cheap but still might be worth bringing to a scrap dealer if one is local.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
![]() Thank you Ted. Ya I cut a small slice off of it and tried to bend it and it broke like pot metal -- so at this point I'm throwing in the towel, lol. Thank you for the info; I did get some knowledge from asking questions here, I appreciate your reply.
Thank you Ted. Ya I cut a small slice off of it and tried to bend it and it broke like pot metal -- so at this point I'm throwing in the towel, lol. Thank you for the info; I did get some knowledge from asking questions here, I appreciate your reply.
Plumber and artist - HUACHUCA CITY ARIZONA USA
A. Maybe Chromium.
Kaktusz Ranch- In the middle of nowhere, California
August 30, 2022
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
Q. Hi Billy ... my name is Redhood. I found a rock with features as yours ... was it platinum?
Red Redhood Urio- Arusha
July 10, 2023
A. Hi.
Billy decided his was not platinum, but probably pot metal (zinc). If you have no instrumentation or chemical analysis, please start by determining the density/specific gravity. You can immediately rule out the possibility of it being platinum unless it's much heavier that most other metals.
Common metals like zinc and iron have a specific gravity around 7. Lead has a specific gravity of 11.34. Platinum has a specific gravity of 21.4 (about twice as heavy as lead), but when impure would still be 14-19 (heavier than pure lead).
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
Separating PGM's from raw ore...at home!
Q. Looking for advice on the best method for separating PMG's from raw ore in the correct sequence to recover the 4 main metals concentrated in the ore.
The ore is concentrated mainly - Pd 68.9%, Ir 8.1%, Ag 6.9%, Au 5.7 %.
Would a simple mixture of Hydrochloric acid and Hydrogen peroxide (20:3) dissolve just the Palladium from the ore or will it dissolve all? Or is there a better way?
My knowledge on refining is very limited. Any advice would be great.
⇐ answer?
hobbyist - Canguu, Bali, Indonesia
October 10, 2017
Q. Hello Dear mining buddies,
Can someone tell me if this material looks like it's got PGM content particularly Rhodium?



We have leased a mine and there's a lot of this type of ore present there. Thanks in advance.
Ali Lateef- Dubai UAE
July 7, 2021
A. Hi,
it will be better if you test your ore in any certified metal testing lab.
refining consultant - Mumbai, India
Q. About 25 years ago I came across some rocks they are really heavy they have a silvery shine and I could see some gold in it. Well I sent a piece to platinum recyclers and they replied what the rock was, along with a $5.00 check. The rock has Rhodium, Platinum and other metals. I have not found anyone to sell these rocks to yet because it is a chemical process to extract. I believe someone could help me refine them. And there is a lot of platinum in them. I have about two tons of this heavy rock. So I was wondering who I talk to to make money.
Chris A.- Easton, Washington
September 30, 2021
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
A. Hi Chris. As Bhupesh has suggested on this page, the best first step is probably to have the material tested by a metal testing lab, which you can surely find with google. This, however, will cost you some money. The next step is probably to find a refinery, again with google.
If you know enough chemistry and have had enough training to proceed safely, you can probably read some refining texts and do at least some preliminary assessment of what you have.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
XRF = X-ray fluorescence test equipment
A. If you will provide me with a dime sized specimen I will run a scan for you without cost. The XRF results will give you an indication of whether or not you have something of value whether or not you should follow-up by sending a sample to a mineral lab for a certified analytical test. Certified tests cost money but they provide you with very precise and detailed info concerning the chemical makeup of your specimen. There are a handful of labs in the western US and Canada that can do this for you.
Kurt Kephart- Billings Montana
August 24, 2022
Q. Hi, I have great reason to believe I have an amount of rhodium ore that could be life changing for my family. I've been desperately trying for the last 2 years to find someone capable and willing to test it but it's been extremely difficult since I am in Arkansas and not many people even know what rhodium is here. Or the facility wasn't able to help because I couldn't afford the test. It's passed every test I've put it through.
It can hold a solid form at temperatures of over 3,500 °F.,
It's nonmagnetic,
Extremely conductive,
Passed the hydrogen peroxide tests,
Has a density like nothing I've ever seen before,
If I was able to send a quarter sized piece to you would you be able to help me with an xrf or xrd test?
⇐ answer?
Young Mother of 2 doing her best - Arkansas
May 27, 2025
privately respond to this RFQ
Ed. note: As always, gentle readers: technical replies in public and commercial replies in private please (huh? why?)
Q. My name is Dean I got two rocks, really heavy, I was told they were rhodium, idk.

- Rover Arkansas
November 29, 2021
A. Hi. Please advise the specific gravity -- it isn't hard to determine. Otherwise, I can think of three choices, but there may be others --
1. Take it to a "We Buy Gold" store and see if they want it, but they probably won't :-(
2. Pay to have it analyzed.
3. Sell it cheap to the person who thinks it is rhodium :-)
Sorry, but I don't think rhodium comes in the form of chunks or nuggets like gold.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
Q. @Ken Vlach. If a person had a bunch of blue Rhodium in his yard what would you do to get rid of that -- just asking for a friend. Thanks.




- USA
January 5, 2022
![]() Hi Glyn. Sorry, but Ken passed away several years ago.
Hi Glyn. Sorry, but Ken passed away several years ago.
Although nothing is impossible, please be careful to not let anyone scam you. Although I haven't done XRF myself, there are many videos which seem to say the testing should be done on pulverized dust because that can be homogenized for a composite reading whereas when looking at a rock you get a different answer each time depending on what spot you're focused on.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
! Hi Glyn,
I am curious as to where your searches have taken you with the blue RHODIUM and other precious metals that the XRF has detected. I'm in the same boat with not much info to proceed with. My XRF
⇦ on
eBay
or
Amazon [affil link] didn't detect but a trace of gold, but was high in all other pgms. I had it retested 3 weeks later with same results. Just curious if you have any insights as to separation, assays.
⇐ answer?
Thanks again.
Hobbyist - Portland Oregon
Q. My husband & I (with kids in tow) went to Vegas for work & family vacation. We decided to try our hand at rockhounding at an old mine near Sandy Valley, Sultan Mine was the name of the mine. My husband found this rock that was very heavy for it's size. After getting home, I errantly decided to scrub it to get it clean without knowing what I was dealing with.


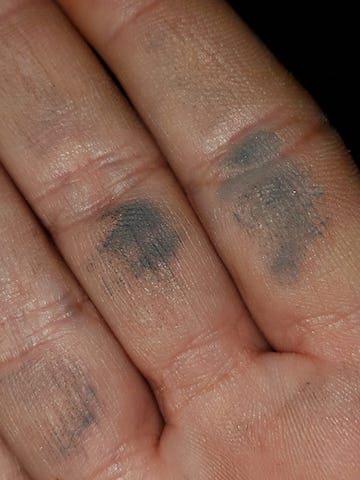

Can anyone who knows metals kind of point out what they think this may be?
Veronica H.Noob rockhounder - Montgomery, Texas
March 19, 2022
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
A. It appears that you probably have lead on your hands, quite literally! Lead of course is toxic and further handling of the rock could cause lead poisoning! I'd recommend using rubber gloves from now on if you must handle it. You could probably take it to a university geology class instructor for further analysis. Also, you can do some research about the Sultan Mine and what minerals are in the area.
Be careful and best wishes!
- Yreka, California
October 22, 2023
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
Q. I have found a weird looking rock which has a unique glassy blue banding in it, somebody suggested it a lunar meteorite.

I am seeking your help to identify this small piece of rock.
Best regards,
Hobbyist - Uttarakhand. India
March 30, 2022
A. Hi Rajesh. Did the person who suggested it is a lunar meteorite have some qualification to make such a suggestion?
On the one hand it's certainly possible that it's a lunar meterorite! But on the other hand I've visited rock museums many times, each with more than 5,000 specimens on display, and every one of which looked really unusual to me ... but each of which were naturally occurring on earth. So, "looking unusual" is no indication of extraterrestrial origin. Sorry, I don't know an inexpensive way for you to find out :-(
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
A. Please send the sample to Physical Research Laboratory, Ahmedabad, Gujarat, India. They have done extensive work on meteorites, lunar samples etc. They would be happy to throw some light on this. You may also contact Geological Survey of India.
Prabhakara HR- Bangalore, Karnataka, India
Ed. note: Thanks, Prabhakara!
Q. I found a stone and/or nugget of what seems to be palladium-rhodium-platinum or meteorite or both -- I don't know. Looking for help on what to do with it ... I'm in desperate need of financial help. If you could point me to some place or person that would help, thank you very much.


Hobbyist - Birmingham, Alabama
April 11, 2022
A. It looks kinda like space nickel. I had some, it was heavy as hell like a metallic grey color. I took a grinder to it to see the cut-away and it was surprisingly soft and hard to really cut because the heat from the grinder kinda softens it. Anyway I gave a friend a piece ... he took it to a pawn shop and got 90 bucks a gram for it. I thought it was palladium, but just nickel from space that was somehow formed in an environment with no oxygen
Dee Snuts [obviously fictitious]- California
September 23, 2022
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
A. Hello Jeremy,
It's very hard to determine metal(s) from a photo. If you want to invest some cash and have it assayed, that would give you a concrete answer. As far as anyone getting $90 a gram for "space nickel" at a pawn shop, I'll be the next one on Jeff Bezos' rocket and have my net out.
- WINSTON SALEM, North Carolina
Q. I have almost 2 lb chunk of something that looks very similar. Can someone help me try to identify it? Wanting to take to jewelry store but didn't want to look like an idiot, so wanted to do as much research as I could. Is it SPACE NICKEL, hoping for at least, but would be nice if was platinum rich or rhodium since I understand it was acquired almost 45 years ago supposedly near a meteor crash that was slammed with platinum. Can you give me an idea please?
Benjamin HIGHTOWER- Nashville, Tennessee
August 10, 2023
Q. Could anyone Tell me what this is.



- Selma Oregon
November 22, 2022
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
? Hello, did you find any answer, I have the same ore. It is NOT affected by any acid, but with potassium chloride ⇦ on eBay or Amazon [affil link] and ammonia ⇦ on eBay or Amazon [affil link] it turns red. ⇐ answer?
Antonio vilhem- Greece
December 11, 2022
Q. Is this Rhodium?



- Glendora California
February 21, 2023
A. Hi Tommy. It's a very pretty rock, but what would lead you to believe that it might be rhodium? I'm no rock expert but I'd guess it's iron pyrite or galena.
While I doubt that anyone can say that there is positively zero rhodium in a chunk of rock based on a photograph, if you picked up ten thousand pieces, the odds of finding a fingernail size sliver of rhodium would still be nearly zero. Rhodium is so rare that even when its value is $20,000 per ounce no one can afford to try to mine it; they only get it as a small byproduct of refining platinum :-(
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
![]() I have the same rock.
I have the same rock.
- Homeland, California
May 17, 2023
A. Looks like the stuff man, I've seen pics similar to the smaller pieces shown, and lead will oxidize in water and is very soft.
SAS Snow- Gresham Oregon
August 27, 2023
RFQ: The assayers I contacted wanted almost $700. Is there cheaper places to get it tested? My nuggets passed the acid testing for platinum and gold; they're dense. I found them coming from earth under a creek and a mineral spring come together with a huge quartz vain running below the same place coming out of the ground looking like huge circle geodes


- Aberdeen ohio
April 1, 2023
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
Q. Hello I need help looking from someone who has knowledge of rare earths and rhodium, I have a property in South America and we have found many interesting rocks. We send an analysis to Canada and we found rare earths and rhodium among others ... but someone interpret these analyzes please. It would be very helpful thank you.



- New York
April 18, 2023
A. Hi J.
Sorry, but those report pages are too small and fuzzy to read. If you send legible copies, we'll post them and see if any public help is forthcoming :-)
...but sorry we can't/don't facilitate private matchmaking. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
This meeting place welcomes Q&As, photos, history, & interesting tidbits.
Please engage with other posters
• When people show interest in each other's situations, the page quickly becomes a fun & informative learning experience for everyone !
• When people show no interest in other people's postings, and just post their own, it often quickly deteriorates into a string of unanswered questions 🙂
Q. Hi, I have a ton of a rock hauled in by a previous miner/owner. The rock is silvery shiny blueish-coppery-silver that is flakey. I don't mind paying for a test of some sort, but don't even know what tests I need done on these rocks. Could you tell me what kind of test to ask for? Where do I send it? And how much of a piece would I send. It might resemble the palladium rock. I understand this was mined off a hillside in the Klamath Mountain Range. Thank you, Gail.
Gail Windyone- Mt Shasta, California
July 29, 2023
Q. Hello
I have found some rocks in a creek bed I have never seen nor can I find anything that resembles them except a space rock. Please if anyone can help that would be great.






- Mexico Missouri
August 1, 2023
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Can someone tell me if this is Rhodium sponge?

- Napoleonville Louisiana
November 21, 2023
A. Hi Jessica.
Rhodium sponge (a manufactured product, not a natural product) is, to my understanding, a foam product made from spongey rhodium powder. This photo looks more like a rock to me.
But even a pic that looks like what you'd expect is a very long way from anybody being able to identify rhodium sponge from a photograph :-)
What is the story? I would certainly suggest an assay before considering a purchase.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
Q. I have a hard time where I am from finding anyone to help me identify rocks so that I can learn what they are what minerals what what type of stone I have no idea so I was going to take a picture of the stone cuz this one's really got me stumped cuz it keeps saying meteorite and I'm pretty sure it's not one but here's the pictures and I found it on the hillside in Columbia Missouri




Hobbyist - Columbia, Missouri
November 22, 2023
A. Hi Stacy. My knowledge of mineralogy wouldn't fill a thimble, so don't rely on me, but meteorite is the last thing I'd suspect about a rock that appears to be a sedimentary one which has occluded other pebbles and rocks into it.
Local libraries, community colleges, adult schools, and universities probably have introductory courses in geology or mineralogy if the topic interests you. If not, you can check online sources for self-study opportunities ⇨
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
Q. I have found a certain metal that looks like rhodium
Can someone help me identify it?
- South Sudan
December 12, 2023
A. Hi Chan,
Rhodium doesn't come in nuggets or chunks, and it's also the rarest metal on earth so it's not clear what you mean by having metal that "looks like rhodium". Can you be a bit more explicit about what you have found and what would lead you to believe it is rhodium?
If you have a chunk of metal and can find someone who has an x-ray fluorescence "alloy sorter /scrap sorter"
⇦ on
eBay
or
Amazon [affil link] (maybe at a scrap yard, a metals warehouse, or a university) they can tell you what metal you have quickl and non-destructively.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
Q. Was just wondering what you think this might be ... this raw form, the second picture is after it was put in a kiln for an hour at 2200 degrees


Any thoughts would be great. Thank you.
Jeff AbbottHobbyist - Cottonwood, California
December 17, 2023
Q. Was just wondering what you think this might be ...this raw form in the second picture is after it was put in a kiln for an hour at 2200 degrees.
Any thoughts would be great, thank you
Hobbyist - Cottonwood, California
December 17, 2023
Q. The rock is 35 lbs, silver white crystals with a green gild tint; the black melted looking metal appears silver with blue tint with a streak on stone to be black. The white crystal has a white shiny streak and the Grey silver deposits have a silver streak on stone.
My question is: if there are 3 metals in rock is that considered ore look magmafic? I'm asking because I want to have it refined to separate the metals but the refinery doesn't do ore, only placer nuggets

- Snohomish Washington
January 18, 2024
This meeting place welcomes Q&As, photos, history, & interesting tidbits.
Please engage with other posters
• When people show interest in each other's situations, the page quickly becomes a fun & informative learning experience for everyone !
• When people show no interest in other people's postings, and just post their own, it often quickly deteriorates into a string of unanswered questions 🙂
Q. I'm having same issues.
Sarahjohn Hitchcock- Willamina
December 20, 2024
? Did you find out what your rock was I have one very similar in looks.
Ginger vanover- Afton ok
February 20, 2025
Q. I have a nugget that has all characteristics of rhodium or palladium; need help knowing what exactly it is.
Jamesy Davis- Oakland California
March 2, 2024
A. Hi Jamesy. If you tell us what those characteristics are, people might be able to help, but people can't suggest tests that you haven't done yet when you don't say which you have done, just "all the characteristics".
What is its density or specific gravity? What tests did you do to determine that it has "all the characteristics of rhodium"? Thanks.
But you will need to have it assayed, which is costly.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
Q, A, or Comment on THIS thread -or- Start a NEW Thread