
-----
Analyze Chrome Plating Bath; Determine sulphates
Quickstart:
For the chrome plating process to work correctly, a small but carefully controlled amount of sulfate is required in the plating solution.
The easiest and most commonly used method of analysis is the "Kocour centrifuge method" which is discussed in detail on this page. Also discussed is the gravimetric method, which is quite a bit more difficult but required by some aerospace manufacturers. Ion chromatography and spectrophotometry are also discussed Read on ...
Q. Hi Angela,
I was wondering if you could tell us the concentrations of the acetic acid and ethanol you used? We have all industrial grade and I don't want it to be explosive.
Lab Supervisor - Westfield Massachusetts
December 5, 2024
by D. Gardner Foulke

on eBay for a song recently (9/24/25)
or Amazon (rarely)
(affil link)
A. Hi Marissa,
Angela's posting is from 20 years ago, so I doubt that she monitors the site or that we can reach her (but we'll try).
Dave Wichern says you don't need to ignite the precipitate, and should not let it boil over.
Concentrated acetic acid offers hazards, and ethanol is flammable, so I think you'll need to establish simple safety protocols. Unfortunately I'm not a lab tech or chemist, and I don't have the D. Gardner Foulke book that Angela relied on; still, I'd hazard a guess that the procedure requires the use of reagent-grade chemicals not full strength industrial supplies.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I am trying to improve the accuracy of my sulfate analysis in chrome plating solutions using the centrifuge method. I am also trying to understand how my results relate to chrome/sulfate ratios and sulfate levels given in various literature.
I originally notice too much variability in my results and found that by increasing my centrifuge time I got more repeatable results. I was then concerned that reading was too low so I started making some standards using virgin chrome with a known amount of sulfate.
I've done this several times and always a reading that is about 7/8 of the amount of sulfate I put in my standard. It is probably worse since there are sulfates in the chrome I'm not accounting for.
I'm thinking about getting some higher purity chromic acid to redo my standardization, but wanted some feedback.
Namely, my chrome supplier is saying that tho the centrifuge method may not be accurate it is the industry standard and I should just read the level on the tubes despite any variability or accuracy issues. Does this seem to be the case? The supplier has a lab with an IC but they use the centrifuge method as well so that kind of makes sense.
I know it may seem small but our aerospace parts have a pretty tight range and the errors I've seen on the tubes could push my results from being right on target to out of range on the high side.
Any insights on this would be appreciated.
Chemist - Oklahoma City, OK
September 23, 2025
A. I want to amend/supplement my response from a few years ago. As I had said, for an accurate reading you need to calibrate your own tubes individually and regularly apply a correction factor (Sharpie the correction factor for each tube right on the glass). It was not uncommon in my experience to find wide variation between tubes even when they are new. Use carefully prepared standards and do everything in triplicate while accurately timing your centrifuge cycle before you determine a correction factor. Tubes that I calibrated were off by up to 16% even when new and I almost always found that the tubes gave a low answer instead of a high one. I repeated this calibration every 3 months, so that I could account for small scratches in the glass left from the repeated cleaning. Once you have calibrated the tubes, you need to be very careful that your samples are ran with the same precise centrifuge cycle times that you used for the tube calibrations (TIME IT, don't just let it keep spinning). I also ran my bath samples in duplicate and averaged the results to correct partially for pipetting variation between samples. All of that being done, I never found any other analytical method (IC, ISE, Colorimetry) to be more accurate than what I could obtain with Kocour tubes.
All of that being said, that level of accuracy is not critical to run a robust conventional chrome process, but nowadays the accuracy of your methods need to be proven and validated in order to pass quality audits. It has almost certainly gone too far to make any logical sense.

Jon Barrows, MSF, EHSSC
Kansas City
⇩ Related postings, oldest first ⇩
Q. We wish to purchase a centrifugal unit for the analysis of sulphate in chrome. Can anyone recommend a product and supplier?
Thanking You,
Kenneth Kelleher- Ireland
1999
A. Kenneth, your best bet for a centrifugal unit is Kocour Co. in Chicago, IL. They have several types of units available.

Jim Conner
Mabank, Texas USA
A. Kocour used to sell an open hand crank unit --somewhat dangerous-- and an enclosed unit with the chemicals and centrifuge tubes.
The only stand alone unit that I am aware of is ion chromatography. This is a bit expensive.
James Watts- Navarre, Florida
![]() Ahh, simpler times ... fragile glass tubes full of toxic, carcinogenic, hexavalent chrome whirling at 1000 RPM atop any handy desk 🙂
Ahh, simpler times ... fragile glass tubes full of toxic, carcinogenic, hexavalent chrome whirling at 1000 RPM atop any handy desk 🙂

Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Suggested RPM and other parameters for Sulphate Analysis
Q. I use a Kocour centrifuge but I am not sure that the results are so accurate.
Does anyone know the recommended type of centrifuge for the sulphates in chrome? (I mean info like RPM, G, swing rotor etc).

Sara Michaeli
Tel-Aviv-Yafo, Israel
1999
A. I think the RPM for the centrifuge should be between 600 and 900 for 3 minutes if using the manually operated one. Kocour has a nice electric one that sure saves your arm if you have more than one or two samples to run.

Jim Conner
Mabank, Texas USA
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. what is the correct "RPM" for the Kocour centrifuge method of testing for sulphates in Chromium baths, I am currently using a 4-tube "Clay Adam" centrifuge...
- SAN ANTONIO, TEXAS, USA
February 13, 2014
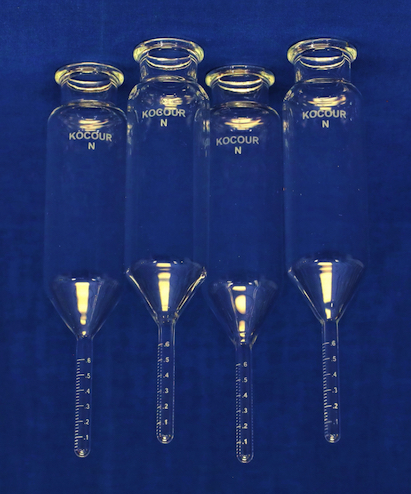
Ed. note: So that readers don't mislead themselves, and so they can better follow the discussion, we note that the Kocour centrifuge method requires special centrifuge tubes (test tubes) with the bottoms greatly necked down, and with graduation marks, for accurate measurements.
A. The only reference I found was in an old British lab book, "Electroplating Laboratory Manual", by Armet, 1965.
They say 1000 RPM for 2 minutes.
- Nevada, Missouri, USA
by Weiner & Walmsley

on eBay (rarely)
or Amazon (rarely)
or AbeBooks (rarely)
(affil link)
A. Thanks Chris. Doing some scouting myself, I see that Weiner & Walmsley ⇨
agrees with that 1000 RPM for 2 minutes .
Dubpernell's Electrodeposition of Chromium from Chromic Acid Solutions ⇦[this on Amazon affil links] doesn't agree or disagree, but stresses that what actually matters is consistency and proving the correlation between your centrifuge results and the actual sulphate content as James Watts, Jeffrey Holmes, Jon Barrows and others try to tell us:

Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. In the dark ages, when I worked in the Udylite lab in L.A., we used a hand-cranked centrifuge made by Kocour, I believe. We kept a bottle of a standard chrome bath with a known amount of H2SO4 to test our techniques.
Chris Owen- Nevada, Missouri, USA
Ed. note: That's sounds like an excellent tip, Chris!
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. Dear Sirs,
We would really appreciate if you could help us on the following problem which we are facing in our labs (Hellenic Aerospace Industry - Physical Science Labs):
In our labs we have to determine Sulfuric Acid in Chromium plating bath. The limits for Chromic Acid in the bath is 225-270 gr/l and for Sulfuric Acid is 2.25-2.70 gr/l (a very small concentration range of 2.70 - 2.25 = 0.45 g/l) . We tried two methods for the determination of Sulfuric Acid in the solution but taking into account the very small limits range of Sulfuric Acid in the solution and the time the methods need, we are not satisfied by the results of both methods
More specifically:
1. The first (titration) method is as follows:
- a. 10 ml sample.
- b. Reduction of hexavalent Chromium of the solution to trivalent with 10 ml Hydrochloric acid conc., 15 ml acetic acid ⇦ on eBay or Amazon [affil link] and 20 ml Ethanol.
- c. Precipitation of sulphates using 15 ml of Barium Chloride.
- d. Isolation of the precipitate by the centrifuge method.
- e. Boiling of precipitate with exact quantity of 0.1 M EDTA and Ammonium Hydroxide (During boiling the precipitate is dissolved and Barium reacts with a part of EDTA.
- f. Titration of the excess of EDTA (the EDTA which did not react with Barium) with 0.1 M Magnesium Chloride.
- g. The formula used is : Sulfuric Acid g/l= 0.98 x 20 ml consumed x M MgCl2 / 0.1.
In above method (A), a difference of 0.1 ml of 0.1 Magnesium Chloride (titrant) results in a deviation of result equal to 0.1 gr/l Sulfuric Acid (that is, a very high deviation of approximately 22% in comparison with the very small limits range of 0.45 g/l of Sulfuric Acid).
2. The second (weighing) method is as follows:
- Steps a ,b and c are the same as above.
- d. The precipitate is collected on a Whatman N 42 filter , is ignited in a tared crucible at 815 °C and is weighed.
In above method (B) we face the difficulty to clean up the filter from the Chromium during filtration. Additionally a small difference of 0.002 gr in weighing gives a deviation of result equal to 0.084 gr/l of Sulfuric Acid ( that is, again a very high deviation of approximately 19% in comparison with the very small limits range of 0.45 g/l of Sulfuric Acid).
We tried to perform above analyses using a higher quantity of sample to eliminate the error, but we faced difficulty during the reduction of Chromium due to rapid expansion (explosion) of the solution during the reaction.
- Here are our questions:
- 1. Have you any suggestions for the improvement of our methods?
- 2. Can you suggest other, more accurate method(s), for the above determination?
- 3. If not, do you believe that above deviations are inevitable, are common to any laboratory and there is no other way to decrease the above mentioned deviations. In this case isn't it strange to ask for results that lie in such a small limits range?
aerospace - Tanagra, Viotia, Greece
2004
by Robert K. Guffie

on eBay (rarely) or Amazon (pricey)
or AbeBooks (rarely)
(affil link)
by Clarence H. Peger

(copies are avail. in select libraries)
Very rarely available from
eBay or Amazon
or AbeBooks
(affil link)
|
|
A. I think that you have set your limits tighter than necessary. Chrome is constantly depleting with plating. sulphate is only lost to drag out, so the ratio is constantly changing throughout the day. The Kocour centrifuge method is fairly quick, simple, reasonably repeatable and more than accurate for chrome plating needs. Use the electric centrifuge and not the hand one. If you like, you can do a correlation of the centrifuge method and your lab method as well as samples from reagent grade materials to establish a correction factor if you really think that it is required. My centrifuge tubes were calibrated in oz rather than grams. They may offer one in grams, but if not it is a very simple conversion. I am a chemist and truly appreciated this adequate method. James Watts- Navarre, Florida A. My method of choice would be (2). That's the standard accepted method. I do not know that barium sulphate would completely dissolve even in a solution containing EDTA, as it is notoriously insoluble in just about anything. If you are determined to avoid the gravimetric method, it might work to fuse the recovered barium sulphate with sodium carbonate ⇦ on eBay or Amazon] , dissolve the melt in dilute nitric acid, then adjust the pH to about 10 with ammonium hydroxide. The EDTA could then be added, then titrated away with your standard magnesium solution. If I were you, I would just do the gravimetry. The boiling over you describe can be avoided by adding the ethanol slowly to the mixture, and heating it gradually. And, it is not necessary to ignite the precipitate. It is good enough to filter it with a Gooch crucible and glass filter mat and dry it at 105 °C.  Dave Wichern Consultant - The Bronx, New York |
|
|
A. A quick and easy way to measure sulphate is precipitation with barium chloride, then centrifuge and measure volume of precipitate. Kocour company makes a centrifuge and graduated tubes for this purpose. It is quite accurate.  Jeffrey Holmes, CEF Spartanburg, South Carolina A. GO FOR IC TECHNIQUES IT CAN DETERMINE sulphates TO ppm levels. Otherwise the techniques you use are the ones commonly used in industry. TRY KOCOUR sulphate testing kits .  Vikram Dogra Irusha India - Chandigarh, India A. 1. The deviation is not much of a problem. There is little or no difference in performance of the chromium plating solution with the small error in the analysis. To do a simple analysis, Kocour Co. provides a Kit that includes a centrifuge that runs at a specified RPM The procedure is simple and quick and may even be more precise than the ones you have mentioned. Sulphate is precipitated by a barium salt in a centrifuge tube with a small diameter tip that is calibrated. Simply read the number after centrifuging.  Don Baudrand Consultant - Poulsbo, Washington (Don is co-author of "Plating on Plastics" [on Amazon or AbeBooks affil links] and "Plating ABS Plastics" [on Amazon or eBay or AbeBooks affil links]) |
by D. Gardner Foulke

on eBay for a song recently (9/24/25)
or Amazon (rarely)
(affil link)
Q. Thank you all very much for responding. Here are some very questions and comments
1. Could I have some info as concerns the supply of the Kocour Kit? Could you describe briefly, what the kit consists of. Is any "Kocour solution" included in the kit and what is it exactly?
2. Have in mind that the hexavalent Chrome must be reduced to trivalent, otherwise insoluble Barium Chromate would form along with the sulphate.
3. The sulphate limits are really tight, but this is because of our clients' specifications.
4. Do you think that the 19-20% deviation in the sulphate determination is a small one?
4. The precipitate has to be ignited at 600 °C because it holds water strongly, as it is suggested in the "Electroplaters Process Control Handbook", edited by D. Gardner Foulke.
Thanks again,
Angela Nikolaou [returning]aerospace - Tanagra, Viotia, Greece
|
|
A. The kit comes with 2 solutions, the centrifuge of choice and special centrifuge vials.You can get the desired information from Kocour or your plating supply vendor. As far as the accuracy of the kit, I will hazard a guess that over 95% of chrome platers use that method as it is sufficiently accurate to maintain a 100:1 plating solution. As I said in my first post, the ratio is constantly changing and it is the ratio that is important. James Watts- Navarre, Florida Q. Hi again, Please give me the address, web site, fax and/or e-mail of Kocour company, to come in contact with. I tried the web site Thanks, Angela Nikolaou [returning]aerospace - Tanagra, Viotia, Greece Their URL is |
A. If you use the Kocour method and want very accurate results, you will want to calibrate the tubes yourself against a prepared 100:1 chromic/sulphate standard. You will find that you will need to apply your own correction factor to most tubes. Repeat the calibration every three months on each tube. This will account for interior scratches that tend to occur with use and cleaning.

Jon Barrows, MSF, EHSSC
Kansas City
Q. We are having Chrome plating plant, for which we measure the temperature and the density of the bath to control the process. However we wish to do a monthly analysis of the bath to ensure everything is OK. Please let us know what elements to be checked and the method of analysis.
Regards,
GEORGE ABRAHAMCHROME PLATING - KUWAIT
2004
A. George,
I hope you have a excellent supplier for your hard chrome solution. The people here at this forum can't not give you the right answer for your hard chrome supplier:). But I like to get a analysis of this, trivalent chrome, sulfuric acid ratio to chrome, iron, copper, and if you use a SRHS Chrome the content's of fluoric silicate.
Regards,

Anders Sundman
4th Generation Surface Engineering
Consultant - Arvika,
Sweden
A. Dear GEORGE ABRAHAM,
You must weekly not monthly make a chemical analysis for your tank and add the chemical compounds if the tank needs that . In chemical analysis you must check chromic acid content and sulfuric acid content and the ratio between chromic acid and sulfuric acid . About the method of chemical analysis you can ask your chemical suppler to give you the data sheet . Also you can daily check the (Be) baumé (it is hygrometer scale for density) for the solution in the tank and compare it to the standard point.
Aly Gomaa- Cairo, Egypt
A. Dear George,
You must check chromic and sulfuric acid content, baumé, trivalent chrome and most common impurities (iron, copper, zinc, etc.).
Regards
Christos Sigalas- Athens, Greece
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. Dear all
I've heard that there is a centrifuge method for determining sulphate concentration in chrome plating solution. However, I don't know how it works and what shall I do. I'll be grateful if anybody could advise.
⇐ answer?
Regards,
- London, UK
2005
Multiple threads merged: please forgive chronology errors and repetition 🙂
How to dispose of these Chromic Acid Test Samples?
Q. At my facility, we hard chrome plate our injection molds for wear resistance and part release. Recently, I purchased new testing equipment to replace our current outdated equipment. I have ordered the following four test kits from Kocour: chromic acid (titration), trivalent chrome (color match), sulphate (centrifuge), caustic strength (titration).
My question is pretty basic but our safety is number one so I'll ask the people who know. I believe everyone here is familiar with the Kocour tests so I'll spare an explanation. I'm new to chroming so the descriptions wouldn't be any good anyway. Once the testing is complete, I have a fairly small sample left over from each test. We have a company that disposes of our waste that will readily accept the tested samples.
Can these tested samples be combined and then disposed of? Combining the samples will make shipping the sample a lot easier. This seems like a simple question but I'm not sure. Again, I'm fairly new to the plating world so any information would be helpful.
- Morristown, Tennessee, US
2005
A. Contact your local sewer authority. Usually there is an exemption which allows a few gallons per day of laboratory waste to be disposed directly to sewer without treatment or reporting.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
A. It is safe to combine all the waste from the chromic acid titration, sulphate test and trivalent test, just chuck them in a old, properly labeled 25L container.
I can't help but wonder what the caustic test is for on an acidic chrome plating solution? it must be to test your cleaners -- this one should be disposed of in a separate container or you will probably end up with a drum full of nasty sludge.
- Melbourne, Vic, Australia
Comparison between Centrifuge Method and Gravimetric Sulphate Test
Q. We chrome plate gun barrel bores. Typical chemistry is 33 oz/gal, at 100:1 sulphate ratio. Due to the thickness required, plating cycle may be 10 hours or more. Our problem is that apparently the ratio is critical to controlling taper in the bores. If we go out of the 90-100:1 ratio, taper becomes excessive. Obviously, we need a more reliable method for sulphate analysis than the Kocour centrifuge test. I know there are gravimetric tests for sulphate -- does anyone have a proven, reliable method to share with me?
George Brackett IIISr Manufacturing Engineer - Saco, Maine
2007
A. I can easily see where the ratio could cause problems with plating control of a gun bore, especially at the top.
My personal feeling is that the Kocour method is very repeatable and accurate if and only if your spin cycle is exactly the same RPM and length of time.
The sulphate ion is a catalyst and is not consumed during the process and the only loss is dragout. Therefore, if you are having a problem in a 10 hour cycle it has to be due to the change in the chrome concentration, which would tell me that you do not have enough replenishment of the plating solution in the gun bore.
The sulphate can be analyzed by a gravimetric analysis method, but it is long and complicated. So bad, that virtually no one will do it. Look at any college level chemistry book for the method. Also, the Metal Finishing Guidebook has the identical method in the analysis section.
I would make up a lab solution of 100:1 plating solution from Reagent grade material, both the chromic acid and the sulfuric acid, and analyze it several times by the Kocour method and then apply a "correction factor", if required, to your analyses of the tank solution. Assuming that you have highly repeatable results, which you should have.
- Navarre, Florida
![]() That was a great response, James. Thanks!
That was a great response, James. Thanks!
My 1998 edition of the MFG includes that section, but my 2012 edition doesn't. You need to look in olde editions --


Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Someone may suggest an alternative method for sulphate analysis, however my experience with the Kocour method is that it is quite reliable. Make sure you are doing it by an exactly repeatable method -- same centrifuge time, etc.
Since sulphate is not consumed during plating, and since you can easily control chromic acid within 10%, why does the ratio get away from your range? If you maintain constant chromic acid concentration, the ratio should change only very slowly as solution is lost to fume scrubber or drag out.
Is something else changing during plating? It is easy to imagine temperature and chemistry changes within the bore unless provision is made for constant circulation of the solution in the bore.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
A. I cannot quote the method from memory. It involves mixing the bath sample, acetic acid
⇦ on
eBay
or
Amazon [affil link] , ethanol, and water, boiling, then adding a good excess of barium chloride solution to drop the sulphate as BaSO4. The resulting mixture is then filtered through a Gooch crucible of known weight, the precipitate rinsed, dried, then weighed.
I once attempted to develop a method for doing this analysis via turbidimetry, a method used for sulphate in water. I had NO LUCK AT ALL. I messed with it for over a year, off and on.
An important source of sulphate in some shops is drag in from the nickel bath that precedes the chrome bath. Good rinsing here is essential.

Dave Wichern
Consultant - The Bronx, New York
![]() Thanks for the responses. Incidentally, we don't nickel plate, so sulphate from drag-in isn't a problem. We initiated a testing method where once a week we run 5 tests on each chrome tank (there are 4). We look at the results, and reason which ones are most likely to be correct. If no two are alike, of course we resample and retest. We use this weekly result for any adjustments needed during the week. Usually we have to cut the solution weekly due to formation of trivalent chrome (we use platinum-clad anodes, and of course the anode to cathode ratio is skewed towards rapid trivalent generation, and the platinum doesn't allow conversion back to hex. We use the weekly sulphate analysis result to rebuild the solution, and so far it's working great.
Thanks for the responses. Incidentally, we don't nickel plate, so sulphate from drag-in isn't a problem. We initiated a testing method where once a week we run 5 tests on each chrome tank (there are 4). We look at the results, and reason which ones are most likely to be correct. If no two are alike, of course we resample and retest. We use this weekly result for any adjustments needed during the week. Usually we have to cut the solution weekly due to formation of trivalent chrome (we use platinum-clad anodes, and of course the anode to cathode ratio is skewed towards rapid trivalent generation, and the platinum doesn't allow conversion back to hex. We use the weekly sulphate analysis result to rebuild the solution, and so far it's working great.
- Saco, Maine
Q. Hi, I'm a chemist that's doing plating for aviation company. Since the specification has been set up by Boeing, I would like to ask how much actually is the deviation between centrifuge method and gravimetric method to determine the sulphate content; the claim by Boeing is that the centrifuge method is not valid to be used for the test method. Regards to the chemistry side, there is no significant difference on the sulphate precipitation by both method but only the byproduct of the analysis? Do you have any idea or research work on that?
I hope you can help me on this.
Regards,
- Subang, Malaysia
2007
A. Sir:
In Hot Dip Galvanizing the flux solution becomes contaminated with sulphate (in plants using sulfuric acid) and a volumetric titration using standard barium chloride is used to determine sulphate. The test takes 5 minutes. It determines sulphate as 0.1%, 0.2%, 0.3% etc. These percents are changed to grams/liter by multiplying by 11.4. This method can be modified by changing the sample size and/or the standard barium chloride solution to attain the required accuracy. A centrifuge is required, which is low cost on eBay .
Regards,
Galvanizing Consultant - Hot Springs, South Dakota, USA
A. Working for Boeing can be difficult at times, especially with some inspectors.
Step 1 is to ask Boeing for a copy of their procedures, including analysis for chrome plating.
If it were me, I would use their procedure and the Kocour method on split samples and track their agreement. Eventually, you will have enough data to ask for a variance in the procedure.
The gravimetric method is a time consuming pain in the rear method that is only minorly better than the Kocour method after you have run it on a bunch of reagent grade standards to come up with any fudge factors required.
- Navarre, Florida
A. Just to add to James' post, we carry out the sulphate analysis by the gravimetric method and it is a right royal pain in the posterior. Add to that that it is a multiple stage process, with each stage open to inaccuracies ... by the time you get to weigh your sample it is no more accurate than the Kocour method, in fact if you are carrying out analysis on several baths then I bet you will end up trying to find shortcuts and introduce new inaccuracies.
Follow James' advice, run concurrent samples by both methods, build up a database of results and offer these as objective evidence of satisfactory analysis.
aerospace - Yeovil, Somerset, UK
A. I have used the Kocour test myself for many years. The key is making sure you add your B solution slowly and that your math in processing your data is correct.
Rodney S [last name deleted for privacy by Editor]industrial plating. - Anniston, Alabama
May 15, 2009
Multiple threads merged: please forgive chronology errors and repetition 🙂
Quick Analysis of Chrome Bath
Q. Is there a procedure available for checking Cr +6, Cr + 3 in chrome bath using spectrophotometer? What is the wave length at which one can detect Chrome?
Kaushik Magiawalaplating shop employee - Gandhinagar, India
2007
A. You may want to get the book Colorimetric Analysis of Metal Finishing Solutions ..." ⇦ on Amazon, AbeBooks by Aubrey Knowles that will describe various methods. If not, you can try a simple direct method with hex chrome at 370nm and tri chrome at 640 nm. Dissolved iron will likely interfere with the hex chrome measurement, but it may be accurate enough for your purposes.

Jon Barrows, MSF, EHSSC
Kansas City
A. The Kocour test is easy and very accurate. Repeatable if you follow the steps accurately. I personally have used it 16 years off and on as they had some others testing the baths over the years. Our company has used it for 20 something years and our tanks stay optimum.
I put the entire testing process on my site, here is the link. Hope it helps:
rodneystephens316.webs.com ⇩
industrial plating - Anniston, Alabama
May 19, 2009
Ed. update: Sorry readers, that link is broken :-(
Please put stuff directly here if at all possible -- we've got all the room in the world and have been a permanent reference site since 1989, whereas nearly everything we link to breaks quickly, so 20 people probably see a broken link for each person who saw it while it was working; trying to maintain links over the decades and a quarter million postings requires an endless, very time-consuming game of whack-a-mole. If you offer a link, please give title & author or other info so we have a fighting chance of fixing it when it breaks, which it soon will.
We thank Rodney, but ... ! We heard the librarian whisper that he's on her "naughty" list for ripping out and swallowing a page from this reference work of ours 🙂
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. Can anyone can post the procedure for solution analysis of chrome.
I would like to check - Chromic acid, sulphate, iron value.
⇐ answer?
employee - Chennai, Tamil Nadu, India
June 2, 2010
Q. I used centrifuge to check for the sulphate content in hexavalent chrome and chrome pre dip. Why is the sulphate content in the pre dip higher than the hexavalent chrome. And what are the standard techniques to check for the sulphate content?
James parker- selangor malaysia
April 14, 2014
A. Hi James. George Brackett seems to have implied that if you do nickel plating the sulphate will run high in the pre-dip. Do you do nickel plating before the chrome plating or not?
This entire thread is about the standard techniques to check for the sulphate content -- can you please review it and phrase your question in terms of the responses already provided so it doesn't just keep getting longer & longer while running in circles? Thanks!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello everyone!
I'm buying a centrifuge for determination of sulphate in chromium bath. I would like to know which one to choose if it's possible somewhere in Europe not in USA?
I have found this one: hettichlab.com/web/global/prod/EBA+200/6455 ⇩
But I'm not sure if it is the best option.
What should be the volume of centrifuge tubes? 50 mL?
- Bovec, Slovenia
October 14, 2014
Ed. update: That link is broken. We try to avoid brand names on these pages (why?), but if anyone has recommendations on generic issues like tube volume, etc., they are very welcome of course.
A. The centrifuge you've posted the link to will not work for that analysis: it's a medical centrifuge and only holds vials that hold 15 mL.
When I formerly worked in a lab doing metal finishing analyses, we used a simpler centrifuge that held Kokour tubes that are specifically designed for the sulphate analysis. for instance, check out this link:
www.kocour.net/productDetail.asp_Q_catID_E_91_A_subCatID_E_92_A_productID_E_92 ⇩
These tubes are glass, hold 50 mL, and have a completely different shape that 'normal' centrifuge tubes.
The sulphate analysis requires 20 mL of sample, 5 mL of 50% HCl, 5mL of 30% BaCl2, so the 50 mL is optimal.

- Seattle, Washington USA
Ed. update 2024: As invariably, that link broke too 
But we've captured a photo so readers can better grasp what Fauna is describing ⇨
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. I have a Koch set centrifuge but have lost the manual. Also need to know what chemicals to use i.e. A and B. It's for sulphate for hard chroming bath. Need good advice.
- Nairobi kenya
September 6, 2018
A. Hi Shahid. The method is explained and discussed here , and at
https://www.hettweb.com/sulphate.html ⇩
and
http://www.plating.com/anapro.htm ⇩
If you tell us the model number of the centrifuge maybe someone can find a copy of the manual. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Ed. update 2024: Yikes, both of these links broke too  , making 7 out of 7 links braking in short order
, making 7 out of 7 links braking in short order
With nearly all plating books out of print, no replacements forthcoming except in Chinese, and broken links everywhere we look, is finishing.com the only English language place in the world which still values reference materials? 🙂
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. I determine the concentration of sulfuric acid in chromium bath using a centrifuge using HCl and barium chloride 20% .
I need another way to determine the concentration of sulfuric acid in chromium bath other than centrifuge.
Thanks in advance.
- Egypt Cairo
July 4, 2021
Ed. note: We put your inquiry on the right page, Hanan.
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. I HAD WANTED SOME PROCEDURE FOR CHROMIC ACID AND SULPHATE DETERMINATION. HOW TO CONTROL SULPHATE AND CHROMIC ACID? PLEASE TELL ME SIR,THANKING YOU.
ASHOKKUMAR MURUGANby George Dubpernell"

on eBay or Amazon
or AbeBooks
(affil link)
September 15, 2021
A. Hi ASHOKKUMAR. You might want to review both this thread and Geoff Whitelaw's article in our on-line library "Standard Chrome Bath Control".
Chapter 10.1 in Weiner & Walmsley [on eBay, Amazon, or AbeBooks affil links] is "Solution Control"; and Chapter 5 of Dubpernell is "Empirical Tests for Catalyst Concentration and Bath Balance".
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Sir,
As I joined newly a chrome plant,
Please provide me the link or something by which I can check concentration of Cr2O4 and H2SO4 and how to set current density in bath and maintain smooth working of process
Employee - Dammam, Saudi Arabia
June 4, 2022
A. Hi Anil. We appended your inquiry to this long thread explaining the methods of checking concentrations.
Current density is more difficult to answer but it is never less that 1 Amp per square inch, usually 50 to 100% more, although often higher.
How to "maintain smooth working of process" is the subject of many large books and tens thousands of Q&A's on this site -- not something that can be dispatched in a couple of paragraphs ... but please give the full situation plus an example of a single problem and readers will probably be able to tell you how to avoid it :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi there,
I recently started working in the plating industry and have been running the analytics for my shop. By analytics I mean conducting solution tests, suggesting additions and related work. I set up a code to take test results as an input (just CrO3 and SO4 concentrations) and it produces suggested additions. Now my code works well and gives the suggested additions under the assumptions that the baths can be modeled as if liquid volumes are additive, and solutes do not change the volume. For example, adding solid chrome will not change the overall volume of the bath, but adding liquid sulfuric acid will. Obviously, this is not true in reality, and the changes in volumes are much more complex than that. These assumptions lead my code's results to be slightly off from what our lab suggests when they send back our samples.
I don't really think it matters that much but I would like to get results that more closely match what the lab sends back so some higher ups in the company will trust my calculations. Anyone have any data or useful constants to model the baths so that I can compensate for changes in volume due to additions better? I am an intern at the company, but I am pursuing a degree in chemical engineering so I believe I can handle more complex answers to this question that may include thermodynamic modeling.
⇐ answer?
- Reno NV
June 14, 2023
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
Q, A, or Comment on THIS thread -or- Start a NEW Thread


