
-----
Coating & Finishing of Titanium for Biomedical
Q. Have been experimenting with corrosion and bio compatibility of materials. Is it possible and/or what is the best way to plate 6-4 titanium with pure titanium?
Thanks
- Clearwater Florida
August 17, 2021
⇩ Related postings, oldest first ⇩
Q. I design implants for spine surgery and I am having trouble with friction and galling between two titanium parts. One part is Ti6Al4V. The other is commercially pure grade 4 titanium (CP4). The alloy has a hardness of 32-36 Rc and the CP4 hardness is 24-28 Rc. Surface pressures are approximately 25 ksi. I want to apply a coating to each surface to reduce the coefficient of friction and prevent galling. The coatings must be biocompatible and resistant to a steam autoclave sterilization cycle. Currently the Ti6Al4V part is hard coat anodized and the CP4 part has no treatment. My initial idea is to hardcoat the CP4 part, and TiN coat the TI6Al4V part. Both processes are biocompatible and survive autoclave but I very open to other idea. My initial ideas have included Teflon coatings, polishing, TiN coatings and the use of other titanium alloys with different hardnesses. Any help is greatly appreciated.
Thanks,
- Austin, Texas
2002
A. Dear Mr. Landry:
Nitrogen or carbon ion implantation has been used with success to address friction and wear issues on Ti alloy orthopedic implants.
Regards,
- San Antonio, Texas
2002
A. Dear Michael:
I am working with the Nanotechnology Laboratory at The University of Nebraska Medical Center. Nitrogen and Oxygen cannot be used, we have addressed this problem.
Regards,
- Omaha, Nebraska
2004
Ed. note: Readers, this forum is for public info exchange. Please share anything you feel free to, but don't hint towards private contact: it disenfranchises the other readers who get to hear only half the story, and it's unfair to our supporting advertisers :-)
A. Sir Michael,
My application is on Titanium 6-4 also, but in a different industry. I suggest that you consider Tiodize II. They will furnish test data.
- Austin, Texas
August 28, 2009
Ed. note: We thank John for this helpful posting from 2009 but times have changed and we can't post vendor/sourcing suggestions anymore ( huh? why?)
Multiple threads merged: please forgive chronology errors and repetition 🙂
Conversion Coating of Titanium
Q. I am looking to coat Titanium similar to a Conversion coating. The coating needs to be .0002 inch thick and olive green in color. The coating must be somewhat flexible as the part flexes in use.
Richard Young- Atlanta, Georgia
2003
|
A. I think that you would be better off anodizing it. This uses a high voltage, up to 200 volts, and the exact color is very voltage sensitive. It does not offer any significant increase in corrosion resistance or wear as it is extremely thin. trisodium phosphate ⇦ on eBay or Amazon [affil link] -TSP is a normal bath material which makes it a relatively non hazardous process and it is virtually instantaneous. Parts identification is the normal use. James Watts- Navarre, Florida 2003 A. Does the coating have to be a minimum of 0.0002 inches (~ 5 micrometers) thick? The reason I am asking is because titanium can be anodized, which results in coatings of various thicknesses and colors. For example, anodizing in a solution of 165 g/L of sulfuric acid for 4-5 minutes at 23 °C and 40 V yields an anodized layer that is olive in color, but only ~ 0.1 micrometers thick. The above data is from Metalast, but Tiodize and General Magnaplate are other vendors who anodize titanium. Toby Padfield- Michigan 2003 Ed. note: We thank Toby for this helpful posting from 2003 (and his hundreds of other helpful answers here) but times have changed and we can't post vendor/sourcing suggestions anymore ( huh? why?) |
A. Anodizing of titanium provides good protection, but it is not very flexible. Conversion coatings would be much thinner than your requirement and less effective. Have you considered powder coating?

Don Baudrand
Consultant - Poulsbo, Washington
(Don is co-author of "Plating on Plastics" [on Amazon or AbeBooks affil links]
and "Plating ABS Plastics" [on Amazon or eBay or AbeBooks affil links])
2003
|
A. I wince when I see much conversation on anodizing titanium. The potential liability and ability to screw up a small market scares me. Jon Quirt- Minneapolis, Minnesota 2003 A. Timadize is also an excellent coating for titanium. Linda Hughes- Santa Fe Springs, California 2003 Ed. note: We thank Linda for this helpful posting from 2003 but times have changed and we can't post vendor/sourcing suggestions anymore ( huh? why?) |
A. Jon,
Perhaps I missed something, but I was thinking conversion coating more along the lines of SAE AMS2486 Conversion Coating of Titanium Alloys Fluoride-Phosphate Type than a coating for biocompatibility. Obviously coatings suitable for titanium implants must meet a stringent set of requirements. Maybe I am being naive, but I cringe at the thought of a person working on a biomedical application asking a generic question over the internet.
- Michigan
2003
![]() The coating is not for biomedical. I would also cringe if this question were for that. It is an industrial application. I have contacted Tiodize about the anodizing and have sent them a sample. I will also be checking into the Timadize. I thank you all for your help and will let you know if I need more help.
The coating is not for biomedical. I would also cringe if this question were for that. It is an industrial application. I have contacted Tiodize about the anodizing and have sent them a sample. I will also be checking into the Timadize. I thank you all for your help and will let you know if I need more help.
- Atlanta, Georgia
2003
|
|
... and the score at the end of eight: Cringers 2, Wincers 1.  Ted Mooney, P.E. Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for instant help or longer-term assistance. 2003 |
Achieving roughness on CP titanium
Q. I am working on titanium CP to make it dull and rough by etching with Hydrofluoric and Nitric Acid bath. What are the bath conditions--acid concentrations, time and temperature?
Thanks a lot.
chemist - Eboli, Salerno, Italy
2004
A. Nitric/HF will probably brighten titanium. To dull the surface use a mixture of 8 oz/gal Potassium Hydroxide + 5% Hydrogen peroxide at room temperature. This will require 1 to 4 hours to produce a dull etched finish.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
2004
Q. I am working with the Titanium CP grade 2 and I have realized the etching with a mixture of 8 oz/gal Potassium Hydroxide + 5% Hydrogen peroxide at room temperature. I have got brown surfaces that I presume to be titanium oxides with impurity of others metal, like iron. I would like to make more biocompatibility the surfaces by modify titanium surfaces in titanium oxides particularly TiO2. What kind of treatment can I test? Tanks in advance.
Rosario Ardia [returning]- Eboli, SA, Italy
2004
A. I have not seen that problem after etching with KOH/H2O2 mixture. Will a simple high pressure remove it?

Jeffrey Holmes, CEF
Spartanburg, South Carolina
2004
Multiple threads merged: please forgive chronology errors and repetition 🙂
Requirement for Titanium Nitride coating process
Q. Dear Sir,
We are in a field of medical technology and we are a manufacturer of Medical implants in India. We got a idea to do titanium nitride (TiN) coating on the surface of medical implants for better biocompatibility. So, please help me for the exact methods to do TiN coating?
Thanks.
- Surat, Gujarat, India
2007
A. You need chemical vapour deposition (CVD) or a similar process.

Geoff Crowley
Crithwood Ltd.
Westfield, Scotland, UK

2007
A. Dear sir,
I'd like to share some points on TiN coatings on medical equipments. TiN coating or titanium nitride coating is mainly done on cutting tools like HSS material, carbide material, etc., to improve the cutting tool life and injection mould life.in machine tools.
There are 2 types of TiN coating: physical vapor deposition (PVD) process and CVD process. If you can give more on your application requirements I can share with you more details.
With warm regards,
- Bangalore India
September 14, 2009
Multiple threads merged: please forgive chronology errors and repetition 🙂
"Titanium Roughening Process"
Q. We have Titanium 6Al4V screws which we would like roughened to increase osseointegration. I have read several papers on Sandblasting with Large grit and then Acid-etched (SLA) but cannot find any information about this on this website or any shops which would do this work. A google search for "SLA implant" brings up lots of hits though.
As I understand this, the parts can be blasted with 200 µm-500 µm Al2O3 particles and then etched in an HCl/H2SO4 solution. Is anyone familiar with this process? Is the acid etching phase related to "pickling" or passivation. I would like to be able provide a very specific description that can be followed.
Any suggestions or comments will be greatly appreciated. Thank you.
engineer - Bozeman, Montana, USA
December 17, 2009
A. There are several ways to activate the surface of titanium implants to create better adhesion to the bones. One of the methods is called "volcano" and can be obtained by creating special porous oxide layer on the surface. There are other proprietary methods to obtain similar surfaces.
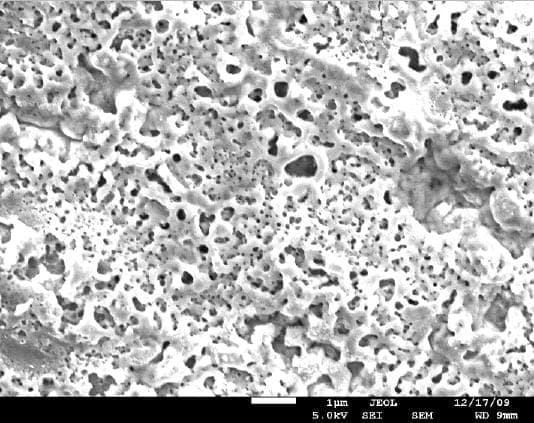
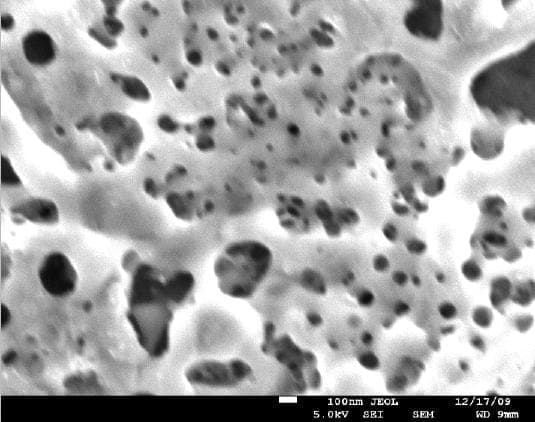

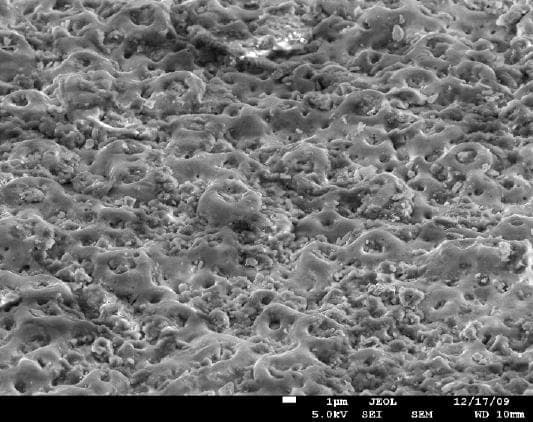
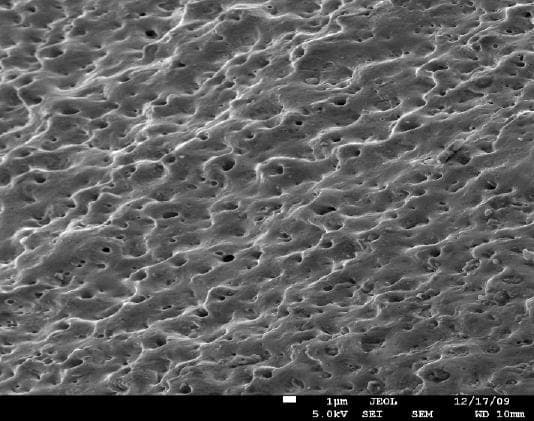
adv.
Contact us for more information.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
Multiple threads merged: please forgive chronology errors and repetition 🙂
"Electrolyte for Titanium coloring for Bone Implants"
Q. I am a mechanical engineer and manufacturing Orthopedic Implants in Titanium in India and want to develop Titanium coloring Plant. Can someone guide me with complete information for the same? I need Specification of Rectifier required and chemical composition of electrolyte required and how to get different colors.
Product Designer - New Delhi, India
August 13, 2011
A. Hi, Ved.
The "coloring" on titanium is not a pigment or patina, it's a clear coating of titanium dioxide that is so thin that it gives the appearance of colors by the same diffraction mechanism as carnival glass or the rainbow sheen of oil on a water puddle. Various colors are obtained by varying the concentration, time, and temperature to build films of different thicknesses. So the coloring of titanium is easy in principle, and tens of thousands of amateur jewelry designers do it, using electrolytes ranging from coca-cola to TSP, and voltages ranging up to 240 V (dangerous, and requires isolation transformer).
But medical implants are not baubles and trinkets! They require biocompatibility, anatase not rutile coatings (thanks, Ling Hao; see letter 7068), and rigorous quality control. You may want to license this technology from a company like Russamer Lab [a finishing.com supporting advertiser], because implants are a serious matter and not a good practice opportunity. Best of luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
August 17, 2011
|
|
! That was one of the scariest questions I've seen on this site.  Marc Green anodizer - Boise, Idaho August 22, 2011 |
Q. In regards to the roughening of titanium alloy implants - in my case spinal screws - Can I ask if anyone can provide further information regarding the performance of the roughened titanium device once implanted? Does the surface treatment change the in vivo performance in comparison to the standard titanium screws? Thanks
Emily SmithMedical Implant - Sydney, Australia
October 24, 2013
A. Emily,
You can purchase the article from http://www.sciencedirect.com/science/article/pii/S1751616107000409 online.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
October 29, 2013
Q. I am looking for an alternative for etching titanium to create a rough surface to apply several coatings.
The current method used is oxalic acid, this is a huge time consuming process up to 15 hours.
We are thinking about H2O with 5% HF, but maybe there are other chemicals we can use.
- Eindhoven, The Netherlands
July 11, 2020
Hi Rob,
adv.
Please contact me directly. We provide consultations on titanium surface treatments and surface preparation. There are several methods on how safely, without hydrogen saturation, to make the titanium surface rough.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
July 12, 2020
A. It appears we are talking about two or three different processes - roughening the surface, increasing the wetability, and developing a porous oxide layer. The pictures presented by Anna Berkovich showing the "volcano" appear to be a thick porous oxide layer deposited by plasma electrolytic oxidation process. The other process is chemical etching which provides a rough surface with a thin unavoidable oxide layer. Both increase the surface wettability.
Rob van den Dungen talks of applying "several coatings" but does not specify the purpose or type of coatings. Emily Smith talks of spinal screws while Baldwin Goodell talks of osseointegration. The two may not be different. But 'biocompatibility' sometimes refers to undesirable bacterial growth on implants.
I hope it may not be out of place to add to the confusion!
Wettability can be achieved by first degreasing and then a 'simple' plasma (argon/air/oxygen) treatment. This is an environmentally friendly physical process. No significant etching is involved in this.
One can excite an electric arc on titanium surface in vacuum so that the arc moves all over the surface randomly removing titanium atoms and creating micro and nano sized craters on the surface. This is one of the PVD techniques to deposit hard coatings like TiN. This gives a clean etched surface. I don't know if anyone has used this physical technique for etching. It is worth trying.
Bangalore Plasmatek - Bangalore Karnataka India
July 14, 2020
A. Hi H.R.
The image of the surface in my previous posts does not refer to this question. The highly developed surface that is attached to my current posting is done by electrochemically etching the surface for future 'Brushete" coating for better ossiointegration. Can be also used for titanium surface preparation before plating.
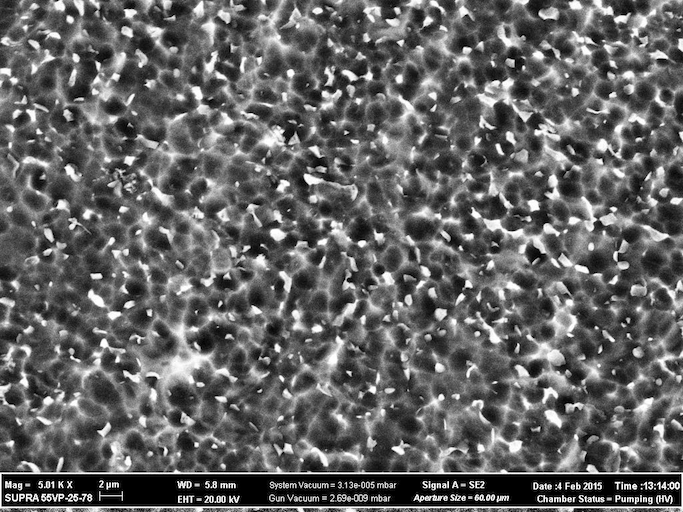

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
July 16, 2020
Q, A, or Comment on THIS thread -or- Start a NEW Thread