
-----
Anodizing 0.003" 5052 aluminum?
Q. My anodizer tried doing a type I anodizing using our rack we made to Chem Film. It is conductive, but not right for the job, fried some nuts off the end of the rack.
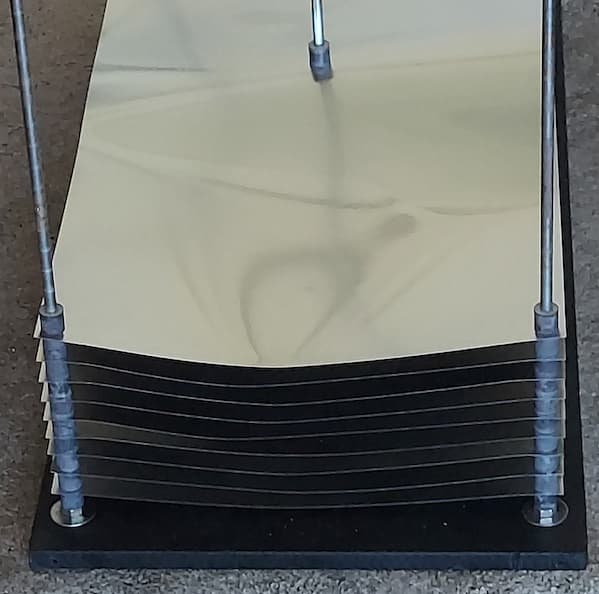

Before I pull what's left of my hair out: can a 11" X 22" X 0.003" 5052 even be anodized? If so, ideas for racking?
Thanks, David
- Palmdale, California
August 15, 2021
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. One at a time, facing the cathode, not stacked like your picture, make contact in at least 4 places, preferably 8 places, ramp slowly from zero up to 16 volts, as the outer edges insulate the current will begin to move inward, go slow, spend at least 5 minutes ramping from zero.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

August 21, 2021
![]() Excellent. Thank you! I am redesigning the rack and will take you thoughts into consideration.
Excellent. Thank you! I am redesigning the rack and will take you thoughts into consideration.
- Palmdale, California
August 21, 2021
A. Uh-Oh, I had missed the "Type I" (Chromic Acid Anodizing), so, amend my comments to. "ramp up slowly to 40 volts, then hold". With the higher voltage, now make contact in more places, one part at a time directly across from the cathode, and in Type I it is important that the cathode be about 1/2 the surface area of the anode.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

August 22, 2021
? What are the nuts made out of?
Chris Jurey, Past-President IHAALuke Engineering & Mfg. Co. Inc.
Wadsworth, Ohio

September 2, 2021
The shaft, washers and nuts are all 304 / 18-8 stainless steel. Spacers are nylon. Top and bottom are HDPE. It would be cost prohibitive to process one sheet at a time, so need to redesign rack to allow anodizing several sheets at a time. In the redesign I can change materials to allow anodizing. I wanted to Keronite the sheets, but they wanted $25 per sheet, so open to other coating suggestions....
David Chaffey [returning]- Palmdale, California
September 2, 2021
A. Steel and stainless steel cannot be used, not only due to poor chemical resistance but because, as the very high electrical resistance anodized film stars building up on the aluminum, all of the current will flow to the stainless steel.
Hopefully your Keronite quoter actually provided a sample, because you really shouldn't try to design a rack for multiple sheets of .003" thick material until you've demonstrated that you can successfully anodize one :-)

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. The only conductive materials that can be used for anodizing fixtures are aluminum and titanium. As you found out, any other metals will be consumed by the process.
Chris Jurey, Past-President IHAALuke Engineering & Mfg. Co. Inc.
Wadsworth, Ohio

September 5, 2021
![]() Thank you!
Thank you!
- Palmdale, California
September 6, 2021
Q, A, or Comment on THIS thread -or- Start a NEW Thread
