
-----
Sodium Metabisulfite and its reaction with water
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. How to remove sodium sulfite in groundwater?
LEIIA RADSTUDENT - IRAN
March 24, 2024
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. Hi everyone,
I'm currently working on a mining project for a gold mine that involves the usage of Sodium Metabisulfite.
Sodium Metabisulfite will be mixed with water to create a first solution at 30% in a make up tank (preparation tank + mixer/stirrer) and after that will be deposited in a storage tank of 12 hours autonomy (it's a batch system), this solution will be dosing to the process not before pass through a post-dilution system (static mixer + water) that will make final solution has 10% of concentration.
The environmental conditions are:
Humidity is around 70-90%
Temperature range: 1 °C - 15 °C
Altitude: 4000 m.a.l.s
My doubts are relating of how to handle properly this chemical powder and its solution.
NOTE: This is the basic scheme of the process:
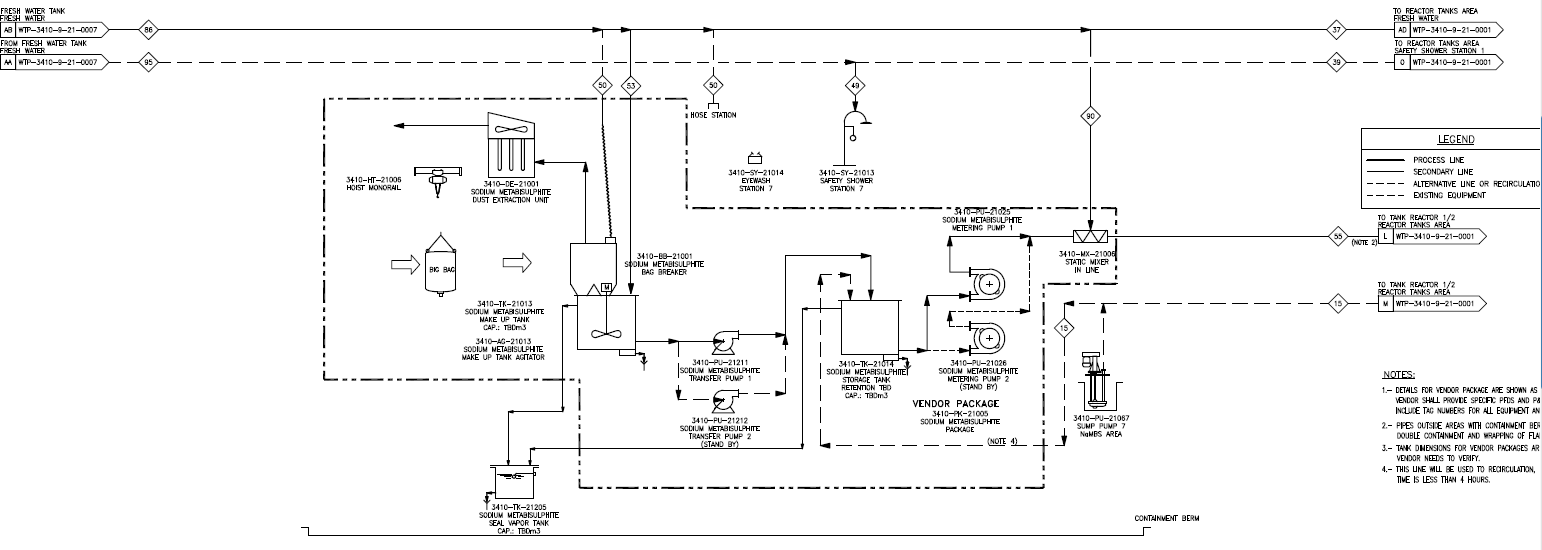
Sodium Metabisulfite contains 65% of SO2 available.
When Sodium MBS get in contact with air becomes oxidized (loss part of its SO2 available)
Due to its hygroscopic properties this powder absorbs the moisture content in the air quickly.
The water they will use is demineralized.
The reaction of Sodium MBS and water is rule by the following:
Na2S2O5 + H2O --> 2NaHSO3
NaHSO3 = Sodium Bisulfite.
However after reading about this chemical it looks like a certain amount of SO2 is released.
The amount of SO2 released depends on temperature, pH, and the % concentration.
So my questions are:
- How hygroscopic is this powder?
- The oxidation process when SMBS get in contact with air, how fast is it?
- Sodium MBS + demineralized water release SO2?
- How much amount of this gas is released?
- How can I control the releasing of SO2?
- Control the Ph level and the solution temperature would prevent detachment of SO2?
- In order to avoid oxidation I read that inject dry air or N2 to the powder before mixing with water would help, but how can I know the amount of Dry air/N2 has to be injected?
- I also read that at low temperatures (just like this case), sodium metabisulfite forms hydrates with 6 and 7 moles of water. Can this affect the reactivity of the powder?
Look at this figure for reference:
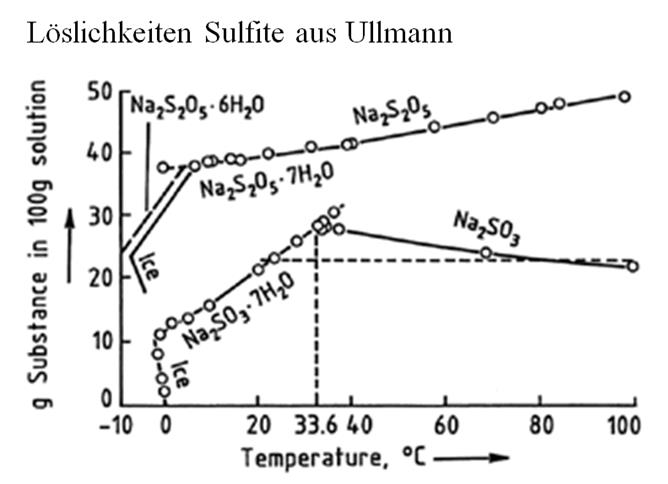
Sodium Bisulfite solution at 30%
This will be contained in a closed tank
- This can also release SO2?
- If Sodium Bisulfite solution get in contact with O2 becomes oxidize (to sodium sulphate), but how fast is this oxidation?
- If there a way to eliminate the O2 inside of the tank?
- What would be the best way to evacuate the produced gas outside of the tank?
- Due to I don't know how much gas will be given off, do you consider a scrubber is required?
- Is there another way to extract this gas from the tank?
I have more questions but these ones are the most important.
It will be very helpful for me if someone can clear my doubts up.
Thanks in advance.
Mechanical Engineer - Peru
August 24, 2014
A. Impressive flow sheet, if your interest is using the SO2 to precipitate gold from aqua regia, Why not use SO2 gas? it will save you a lot of steps and at the end you have much less spent solution to treat.
I normally would think that your chemical supplier should have a lot of your question answered.
Good luck,
- Mays Landing, New Jersey, USA
A. I've used SMBS for 5 years now (Zn depression in Cu circuit). It's hard to use this product and not produce SO2. We get it in 1200 kg FIBC bags with an inner liner and still have problems with gas production in hot humid days via hygroscopy. The mix and holding tank have to be sealed and vented or you will gas out the tank room. And you can expect rampant scaling of all slurry handling equipment downstream. Research this product thoroughly before implementation.
Jim Blairmetallurgy - Timmins, ON, Canada
December 11, 2018
|
A. Probably the majority of people in the chrome plating industry (and those platers still using hexavalent chromates) use SMBS to reduce hexavalent chromium to trivalent. I realize that this is not quite responsive to the question about using it for gold refining, but I mention it on this metal finishing site because every approach has advantages & disadvantages and I would not want readers to think it is excessively dangerous or non-useful for reduction of hexavalent chromium. In the plating industry we tend to use smaller amounts (often purchased in 50 pound bags rather than 1200 kg) and higher dilution ratios. It does evolve fumes, but if the mixing tank is simply equipped with a loose cover, and if it is applied in treating wastes that are closer to pH 5.0 than 2.0, the smell & fuming are not major issues. Regards,  Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for instant help or longer-term assistance. A. Hi Ted  Geoffrey Whitelaw - Port Melbourne, Australia
Regards,  Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for instant help or longer-term assistance. |
Q. Hola Alejandro and Everyone,
Very well explained question. I'd like to know how did this go and what are your conclusions?
In my application I'm interested in dissolving SMBS for production of sodium bisulfite for water treatment. The release of SO2 is unwanted, and I would like to know if the release of SO2 can be avoided if dissolving in a neutral or high pH environment.
The concentration required is 40% w/w of SBS.
Any information is welcome.
- Bristol, UK
March 11, 2019
A. Hi Bruno. I am not familiar with using SMBS at anywhere near that concentration; why does it need to be so concentrated in a water treatment situation? But Wikipedia offers data indicating that it is very highly soluble, so mixing it at that concentration sounds possible.
I am also unfamiliar with adding alkali to the mix to try to limit SO2 generation during the holding period. It might work! But the main release of SO2 comes during the acidification. The formula, shown in Wikipedia and elsewhere, is:
Na2S2O5 + 2HCl => 2NaCl + H2O + 2SO2
In water and wastewater treatment, it is this SO2 that provides the reducing power. I don't know why you are using SMBS but it won't perform a reduction process unless the pH is low enough to release the SO2 gas. Reiterating my personal experience with the use of SMBS for reducing hexavalent chromium to trivalent, I found that a long retention time allowed reduction with a reasonably high pH (about 4 - 5 if I recall correctly), and very little evolution of SO2 gas into the air; whereas a lower pH allowed a much quicker reaction but with far more release of SO2. Please tell us why you are using SMBS so we can understand your needs better.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I am using SMBS as antioxidant for Manufacturing of API. But when added as powder 40% of reaction produces an Impurity. Please advise how we can avoid this formation of Impurity.
Sakina Sitabkhan- Mumbai, India
March 13, 2019
A. Hi Sakina. Sorry but I don't know what you mean by API, or whether there is a need to use 40% concentration SMBS when making it, or what impurity you are referring to :-)
It sounds like you are implying that that this high concentration of SMBS causes some kind of reaction that produces an impurity that is not produced when SMBS is used at a lower concentration? But I don't understand what kind of help you are looking for :-(
Please spend a couple of paragraphs so we understand what your question is. Thanks!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Hi Sakina, I'm not normally on this website but stumbled across you question and may be able to help. I am in the water treatment chemical industry and use sulfite containing solutions. According to my notes, SMBS has a maximum solubility of approximately 38%. Having said that, in solution where the pH exceeds 8.5, the bisulfite forms sulfite, which has a maximum solubility of roughly 10%, meaning everything over that will precipitate and end up as white crystals in the bottom of the container. In my opinion, reduce the SMBS to 38% or less and make sure the pH does not exceed 8.5.
Hope this helps.
- Chico, California, USA
March 13, 2019
Q. I'm looking at the possibility of treating surface water (from a river) with either Sodium bisulfite (SBS) or Sodium Metabisulfite (SMBS) for the purpose of removing dissolved oxygen. This is for a water re-use project, where the D.O. content is of particular concern. Does anyone know if the SMBS or SBS would be a better option? I know SMBS + water produces SBS, and is typically less expensive. I'm just curious what the byproduct(s) might be, and also what the basic mixing ratio is (which I'll need to scale up or down based on the water quantity being treated). On a side note: the river water will be pre-filtered for larger materials (algae, etc.), but won't undergo any other chemical treatment besides what I've already discussed for the D.O. Thanks in advance for any and all insight/input.
Sammy SmithHydrogeologist - Land O Lakes, Florida - US
June 27, 2019
Q. Thanks for useful chemical comments on the above topic. I was wondering if anyone can guide me on SMBS make-up from powder to SMB liquid 38%.
What I understood so far the main issue would be release of SO2, but controlling pH and temperature would help out to control the release, although I was wondering how to keep a Food Grade when blending the water with SMBS.
Thanks,
Lyazzat
- Peyruis, France
July 7, 2019
Q. Some steel raw material which I use got contaminated (during transportation) with some whitish/yellowish substance which I later learnt that it was Sodium Metabisulfite.
I would like to get rid of this substance from the steel raw material. What can I use to neutralise and dispose it of with minimal effect on the surrounding environment?
- Kalumbila, Zambia
February 4, 2022
Q, A, or Comment on THIS thread -or- Start a NEW Thread