
-----
Coating thick copper on copper base metal; using plating to cold weld items together
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Clemence, can you please explain more about your electroplating way to get 1 mm thickness.
Tharwat Ismailhobbyist - Alexandria
May 12, 2023

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Tharwat,
Clemence's posting was from 12 years ago now but we'll try to contact him.
Please tell us your own situation. Thanks.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. What I did was actually an electroforming. Nowadays, you can find many well-documented videos on youtube about it.
Clemence Ronald- Bali
May 14, 2023
⇩ Related postings, oldest first ⇩
Q. Is that possible? I need to plate/coat a small recessed rectangular area (3mm x 3mm) in the PCB to make it level with the PCB's surface. It's a thermal junction for an LED heat sink.
My preferred technique is sulphate. All of my first attempts ended up with no cohesion at all. I use CuSo4 + NaCl + distilled water. I ran the current using old Nokia phone charger.
Many thanks in advance
Hobbyist - Indonesia
October 12, 2010
by N. Kanani

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi, Clemence. What you want to do isn't impossible, but might be impractical without experience laboratory-quality equipment. Points to work on:
• electroplating "wants" to do the opposite of the leveling you seek -- it wants to grow a high spot and concentrate the plating buildup there. "Brighteners" can help.
• a thickness of 1 mm, while not unprecedented, is thick for a hobbyist.
• chlorides are very powerful stress inducers, and probably incompatible with plating of that thickness. sulfuric acid is good, NaCl is not.
Regarding poor adhesion: what are you doing to prepare the copper base metal? The plating must deposit onto clean, raw, active copper, not onto dirt, copper tarnish or oxide.
Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Finally I've succeeded to plate the copper using a simple Nokia charger, CuSO4, distilled water, H2SO4, and some sugar. You were right, sir, the cleaning process is very important. I cleaned the specimen using sandpaper, NaOH, and finally soaked it for 5 minutes in H2SO4 before the immersion. The plating is thick enough with fairly good cohesion, nearly 1 mm thick! But after reaching a certain thickness it started to develop coarse granules. I should find a brightener agent.
Finally I've succeeded to plate the copper using a simple Nokia charger, CuSO4, distilled water, H2SO4, and some sugar. You were right, sir, the cleaning process is very important. I cleaned the specimen using sandpaper, NaOH, and finally soaked it for 5 minutes in H2SO4 before the immersion. The plating is thick enough with fairly good cohesion, nearly 1 mm thick! But after reaching a certain thickness it started to develop coarse granules. I should find a brightener agent.
The picture: [Ed. note: the imageshack graphics do not seem to be available]
The copper tube was only 0.5 mm thick
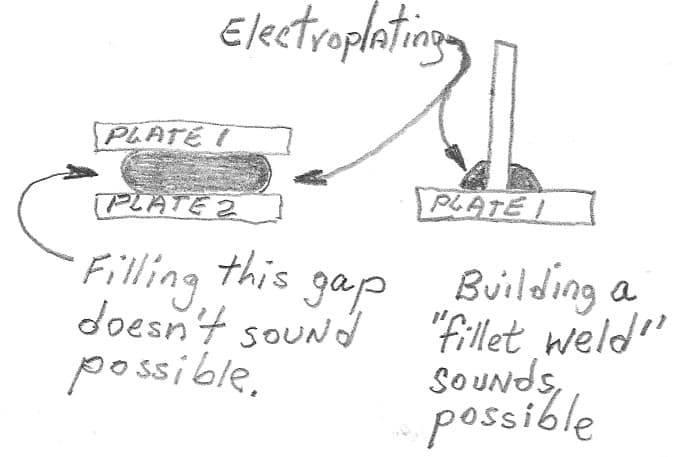
Q. I'm thinking to "cold weld" the LED thermal pad directly to copper heatsink using electroplating. Is that possible?
- Indonesia
A. Hi again. From my understanding of how electroplating works I do not believe it is possible to build a plating layer between two parallel surfaces to 'cold weld' them together, and I've personally never heard of it.
But I don't see any theoretical reason that you could not use electroplating to form fillet welds, although their strength might be insufficient for many practical applications.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread
