
-----
Electropolishing and Chemical Etching Processes for Cobalt Chromium
Q. Hi, how are you my friends? Please help me. After electropolishing 20 or 25 pieces, the contact point of the workpiece with the positive electrode turns gray and spreads throughout part of the piece, making it look very ugly.
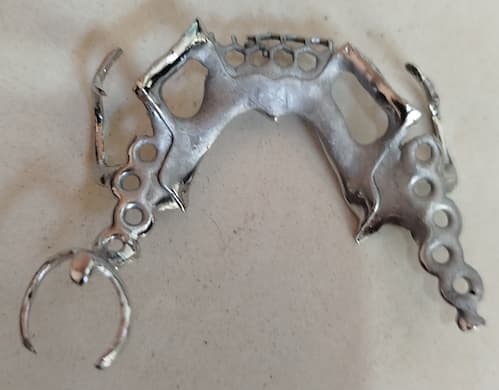
Why does this happen? Can you help me? I am polishing prosthetics with the formula provided by Bruce at 30 degrees, DC 12 volts for 15 minutes. Thank you very much.
Ricardo Lorenzi Bertolo- Ribeiréo Preto
July 4, 2025
A. Hi Ricardo. I find it hard to believe that anything is "spreading" from the contact point to the part in the fashion of a disease. Grayness is sometimes burning due to excessive current, but is more likely etching due to insufficient local current density, or too little agitation, or excess water. The tips apparently are stainless steel, and when they turn gray perhaps they have somehow become worn to the extent that they no longer make good contact and carry sufficient amperage? If that's not it, are your racks coated with plastisol (or just bare copper)? If plastisol coated, might moisture be dripping out which is causing etching?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇩ Related postings, oldest first ⇩
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. I am looking for any and all information on surface etching of cobalt chrome alloys using an ambient temperature bath.
David B [surname deleted for privacy by Editor]1996
A. More words please. Sorry, but it's just not clear whether you want to etch a cobalt-chrome alloy . . . or whether you are suffering etching when trying to electropolish it . . . or quite what your situation is. Do you require an ambient temperature bath, or you are saying that the bath you are using which is causing the etching is ambient temperature.
The most common causes of etching are excess water (and at ambient temperature you have no evaporation to deal with the drag-in) and insufficient current density.
The Electroplating Engineering Handbook ⇦ this on
eBay,
AbeBooks, or
Amazon [affil link]
has a good chapter on electropolishing, and Metal Coating Process Corporation runs an electropolishing school. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I am currently undertaking research investigating the use of electropolishing (used in CoCr metal denture manufacture) in enhancing the fit of ceramometal copings or substructures (Porcelain Fused to Metal substructures) after they have been subjected to extra expansion during the investing stage before casting the wax patterns in wilceram metal. Any information on like or similar research or related information on electropolishing will be deeply appreciated.
Tassy Pillay- Umhlatuzana, Durban, South Africa
2002
A. Hi Tassy.
Although I am not personally familiar with the specifics of electropolishing of CoCr alloys compared to other alloys, the Electroplating Engineering Handbook ⇦ this on
eBay,
AbeBooks, or
Amazon [affil link]
has an excellent 20+ page chapter on Electropolishing which explains both the whys and the hows of the process.
Metal Finishing Information Service / Finishing Publications Ltd. in Stevenage, Herts, England has a specialty computerized literature search database for the metal finishing industry which can be accessed online, or is available on CD; they also can perform the literature search for you if you prefer; and scholar.google.com can usually find such stuff.
Russamer Lab [a finishing.com supporting advertiser] has done a lot of similar work, I believe.
Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I need information regarding electropolishing of Cobalt Chromium. What would be the best electrolyte to use? I know the process of polishing stainless steel quite well. Is there a big difference in polishing Cobalt Chromium compared to polishing 316 SS? Any form of help would be greatly appreciated.
Arthur Marsden- Cape Town, South Africa
2002
A. In order to electropolish cobalt you either use strong acids with conventional method or environmentally safe salty electrolyte with special high-voltage equipment. Look at the pictures on our web site to see sample of such equipment and some polished cobalt parts.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
Q. Anna, a question, What do you mean with high voltage? Do you think
20 volts is high enough for electropolishing cobalt chromium? I have tried till 16 volts but I have not succeeded.
Best regards
medical device - Spain
July 2, 2010
A. Sorry Isabel, but I cannot reveal this technological process since it has been licensed exclusively to one of the medical implants manufacturing companies.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
July 6, 2010
Q. Dear Sir,
I want to electropolish cobalt-chromium alloy. I am already dealing with stainless steel electropolishing. I want to know which electrolyte should be preferred to electropolish cobalt-chromium. For better and smooth finishing, how should the parts be precleaned?
Vijay Nathmetal finishing - Alahabad, UP, India
2003
A. In the dusty archives we see this response in Spanish from Tom Pullizzi's mother, Vijay:
No quiero nada por nada
No te lo quiero tomar
Qué por entendido tengo
Qué él que toma-tiene que dar.
Your inquiry will probably enthusiasm if you briefly describe your experience with stainless steel electropolishing, including the electrolyte you use. Thanks!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
? Dear Mr. Mooney,
An english translation Please? expectantly,

Khozem Vahaanwala
Saify Ind
Bengaluru, Karnataka, India

![]() Poems lose a lot in translation, but it basically says, "Yes, I'm asking for help but I wouldn't presume to do so unless I was willing to help in return." The point being that postings which ask for help but are cast in a way which offers something in return often draw enthusiastic responses.For example:
Poems lose a lot in translation, but it basically says, "Yes, I'm asking for help but I wouldn't presume to do so unless I was willing to help in return." The point being that postings which ask for help but are cast in a way which offers something in return often draw enthusiastic responses.For example:
"I electropolish stainless steel in a solution of ______ at _____ degrees and _____ASF. I pretreatment it by going through a ____oz/gal caustic electrocleaning tank, and I get good results! "Now I need to electropolish cobalt-chromium. Is my solution okay for cobalt-chromium? Should I use the same current density? Is electrocleaning sufficient pretreatment, or do I have to activate the material in some way?"
But "I know stainless steel electropolishing; tell me how to electropolish cobalt-chromium" often don't draw a response.
I am unfortunately unable to help this particular poster technically myself, so I'm trying to get people help based on my experience with moderating the site :-)

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. Dear Sir,
I need a solution to electropolishing a Co-Cr alloy (51:20 chemistry value). A Glycol, Sulfuric Acid, HCl has been suggested, however does anyone know of any other suitable solutions and of a more suitable ratio of given chemicals.
Which glycol is more suitable?
Can you guide me for sources?
Thanks in advance. Regards, Devesh
Devesh Kothwala-Medical - SURAT, GUJARAT, India
2003
Q. I need to know a couple of pieces of information about electropolishing. First can CoCr be electropolished and if so what kind of bath is needed. Secondly we currently electropolish stainless steel, if we put the CoCr in the same bath will there be any contamination between the parts? This is a big issue due to the customer we deal with. If you have any information about this situation please let me know.
Scott Hawkinsmedical instruments - Dayton, Ohio
2004
A. Hi Devesh. Again, I don't know the idiosyncrasies of Cobalt Chromium alloys, but there is an old U.S. Patent, No. 2,607,772 which explains a sulfuric-glycolic mix for electropolishing stainless steel which may be applicable for you.
I believe that a plating process distributor may be able to offer you a proven proprietary. Alternately you may wish to contact an electropolishing specialist which offer training in the specialized chemistry.
Hi Scott. Surely Cobalt Chromium alloys can be electropolished. Obviously your second question about cross contamination is difficult to answer conclusively but considering the mechanism and polarity of the operation, I don't think I foresee any contamination in the electropolishing step per se.
Best of luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. Hi!
What solutions to use to Electropolish Co-Cr Alloy (L605)?
Could you share your Co-Cr electropolishing solution recipe here?
Thanks
- Warsaw, Poland
2006
Ed. note: Some have already been shared, others are shared later ... read on :-)
Does electropolishing of Cr-Co dentures effect the thickness, and how?
Q. I'm studying dental technology and would like to know more to have less errors.
Thanks
student- Jordan
2006
A. Hi Nancy. Electropolishing selectively dissolves the asperities because the highest current density is there. If the surface was reasonably smooth before electropolishing, I think very little metal needs to be consumed to achieve the smoothing. Here's what Dean Ward had to say about that in the Electroplating Engineering Handbook:

Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Post cleaning of Co-Cr alloy type L605
Q. Hello
I am working with a Co-Cr alloy, L605, which contains 15% Tungsten. After welding process of the alloy, followed by electropolishing treatment, I receive impurities with mushroom shape (approx. 5 µm size) on the surface of my alloy. I tried to remove it by Chemical etch, mechanical polishing, ultrasound, sandblasting, nothing was helping.
I would really like to have advice on what I should do? I think thermal treat can help, but don't know what temperature and time.
I will appreciate any help.
Thank you,
microprocessors - Italy
2006
A. The possible cause of this problem is bad preparation of the surface before electropolishing. After welding there were developed oxide layers of different thickness. In locations where oxide layer thickness is max (and oxide does not conduct electrical current), electropolishing does not take place, but electropolishing does go on around such locations. That is why you see creation of the mushroom shape.
You need to add etching step after welding and before electropolishing.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
![]() Thank you!
Thank you!
microprocessors - Italy
Q. Hello, I'm doing a M.S. thesis work on electropolishing Co-Cr alloy L605. I hope this question hasn't been asked yet. I don't know what solutions to use to EP L605. I read through the standard guide for electrolytic polishing of metallographic specimens by ASTM. It indicated a couple of acids that are used for SS, and other metals. But I was unable to find a specific acid for Co-Cr alloy L605. What are the appropriate solutions that I should be using to EP L605. Can I still use the solutions indicated with SS 316L and still have the same effect for L605?
Hokuto AiharaSan Jose State University - San Jose, California, USA
2007
Q. Hi, I have the same question. I have looked and spoken to many people, but no one has been willing to help in this regard. Most people say it is proprietary and leave it at that. I also wanted to know what metal is generally used for the Cathode? Any feedback would be greatly appreciated.
Iain Kemp- South Africa
August 18, 2010
A. Most electrolytes for Cr Co alloys are composed of:
85% EF 200 Ethylene Glycol,
10& Sulfuric Acid,
5% Hydrochloric acid. Brilliant Shine. Some corrosive fumes.
(The hydrochloric acid can be optional).
-OR-
85% EF 200,
15% Phosphoric acid (Shine varies from Brilliant to matte)
This particular formula has good effect on most CrCo alloys and has a 70 year history in industry.
Technical Product Manufacturer - Central Point, Oregon, USA
December 4, 2013
Etching solution for Co-Cr L605 Alloy
Q. Our company manufactures medical devices, coronary stents.
Now presently I am looking for stent Descaling Process (to remove the metal oxide created after Laser Cutting), Annealing (to refine the grain size, hardness and elongation), Electropolishing (for bright & rounded edges) and Etching (To develop the microporosity on the stent surface) processes for chromium cobalt L605 alloy.
Regarding Descaling, Annealing, Electropolishing, I have validated all processes as per our application but after polishing we need to do etching. Here I want to do microporous development after etching on the stent surface but so far I cannot achieve.
I had taken different composition of HCl, H2SO4, and DI water with different times & temperatures, and also FeCl3 10% 20% 30% 45% solution and given various times and temperatures but I cannot develop microporosity on the stent surface.
Please give me suggestion what can I do for further?
Thanks,
Regards,
Manufacturing Cardiovascular coronary stents & support devices - Surat, Gujarat, India
January 30, 2008
A. Hello,
Use an electrolytic etchant (10%, 2 sec, 3 mv) such as: oxalic, phosphoric, etc.
- Minneapolis, Minnesota, USA
Electropolishing Solution for Platinum Chromium stents?
Q. I am currently involved in research and development of platinum chromium coronary stent. Can you suggest which acids and chemicals are involved in Descaling (Acid pre-cleaning process) and Electro-Polishing Process?
We are already engaged with Co-Cr l605 and SS 316L coronary stents.
I certainly appreciate your fast help.
Looking forward to hearing from you.
Regards,
Manufacturing Cardiovascular coronary stents & support devices - Surat, Gujarat, India
May 12, 2011
Q. Dear Sir,
I have found crack problem in Co-Cr L605 metal in coronary stents after Electropolishing, I have reduced laser power during cutting, variable focal points, gas pressure, but not reduced this crack problem in coronary stents.
Regards,
- Surat, Gujarat, India
May 21, 2013
adv.
Russamer Lab licenses electropolishing of the Cobalt Co-Cr stents to interested companies. Contact us for testing samples arrangements.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. In electropolishing CoCr, why not use Co in anodic and cathodic chemical reaction?
- Ahmedabad,Gujarat, India
April 1, 2014
Hi Kinjal. Sorry, I don't understand you. Do you mean: "why not use Cobalt cathodes?" Please explain your situation. Thanks.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Cathode and racking material for electropolishing of CoCr alloy?
Q. Hello Sir,
I want to do electropolishing of CoCr L605 material.
Will you please suggest me the Material for Anode and Cathode?
- Gandhinagar,Gujarat and India
April 18, 2014
A. Final finishing of Co-Cr stents faces the following challenges:
1. The surface must not be saturated by hydrogen, in order to prevent embrittlement;
2. Since Co-Cr stents are usually very thin, special holder design is required to distribute electrical current evenly;
3. Depending on the alloy composition there may be various hard-to remove molybdenum or other carbides, that do not disappear during electropolishing.
We work with cobalt-chrome material for almost 15 years, mostly with knee implants.
adv.
Our ultrapolishing method allows to remove all of the peaks of hard-to-remove carbides. However for such delicate stents the ultrapolishing method does not work.
Recently we developed two-step processing. During the first step the surface is smooth electrochemically --

(image of the stent)
The second step dissolves all carbides on the stent surface.

(SEM image)

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
May 3, 2014
Q. I want to know etching solution composition for CoCr L605 Material.
Can you describe it?
- surat, gujarat, India
December 13, 2014
Q. What is chemical etchant for Co-Cr alloy L605? Which electrolytes can be used for above?
shrikant thorat- Pune, India
December 16, 2014

A. Hi Dhavel; Hi, Shrikant.
Please try to phrase your question in terms of the previously posted answers to keep the dialog moving forward.
Thanks & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi sir,
I need to know about the sticking problem occurred after annealing process in CO Cr L605 alloy? How to get rid of it?
Is there any solution to separate the material?
Kindly guide me.
- Surat, Gujarat, India
August 8, 2017
? Hi Vickysh. Unfortunately, "the sticking problem" doesn't mean much to me. Are you saying that you performed an annealing process, and the parts fused together? If these are stents or other medical or dental parts, I'd find it hard to believe that they could be salvageable after they have fused :-(
Luck and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. What kind of surface texture is created after etching of Co-Cr alloy and why? Example, like formation of porosity on stent surface? What are research journals available for the etching of Co-Cr alloy?
Shrikant thorat [returning]- Karad, Maharashtra, India
February 13, 2015
Q. Dear Sir/Madam
Would you please let me know how can I measure the current density for various voltages during a given electropolishing process?
Thank you for your answer
- Isfahan, Iran
March 11, 2015
A. Hi Aref. The average current density is easy: it's just the current divided by the surface area of the parts.
But the specific current density at different locations in the tank is quite a bit more difficult. There were immersible current density monitors available from companies like QCI but I'm not sure if anyone manufactures them anymore. You could try Material Testing Technology in Wheeling, Illinois. Stresstabs may help too. You can also research "electrochemical modeling software". Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi sir, I'm a biomedical engineer. Currently I work with electropolishing machine for Chrome - Cobalt alloy (L605 alloy). However, we can't get the good surface after polishing because we don't know the detail of polishing liquid. What kind of acid I should use? Sulfuric acid or Phosphoric acid and the concentration to get the best result? Can you give me some advice, thank you very much.
BAO NGUYENbiomedical engineer - Ho Chi Minh City, Vietnam
May 4, 2016
![]() Hi Bao! Bruce Dean has taken a first cut at that. Please ask for clarification, or describe in what way it didn't help.
Hi Bao! Bruce Dean has taken a first cut at that. Please ask for clarification, or describe in what way it didn't help.
We might also suggest that you contact Russamer Lab [a finishing.com supporting advertiser] if you'd like to purchase appropriate chemistry or consulting services; and if you want technical references on the subject, we've listed books, computer databases, and patents covering it on the above page.
Good luck, and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi everyone. I work with electropolishing of CoCr alloy for medical implants. We work with a solution containing sulfuric acid (4%) and phosphoric (13%) besides others components. Do you have some suggestion to control the stability of the electropolishing solution? Or perhaps some other acid solution to deal with this specific alloy?
At some stage of the polishing (after 100 pieces polished) the CoCr alloy begins to show defects, even using the parameters that provide a good surface.
medical - Goias, Brazil
November 24, 2016
Q.
We make CoCr stents which are then electro-polished using EP solution and machine supplied by ESMA, USA. The problem we see, at the rate of about 20% fallout, is pits and cavities on the surface of the stents.
We are not sure if the pits/cavities are the result of impurities within the CoCr raw material, or the result of something happening in the EP process or prior processes (which include acid descaling and heat treatment)
Would appreciate any help identifying the source and suggestions for what we could do to reduce the fall out. Thanks in advance ...
best, Anil
- HYDERABAD, India
December 13, 2016
A. There are many ways to electropolish CoCr, the effectiveness depends on the exact alloy composition and alloy supplier. Another problem is that even after regular electropolishing, there are pits produced by undissolvable carbides. We remove them by final plasma-like electropolishing, with duration of few seconds.
adv.
Contact me for more information and testing possibility. I have attached SEM images before and after plasma treatment
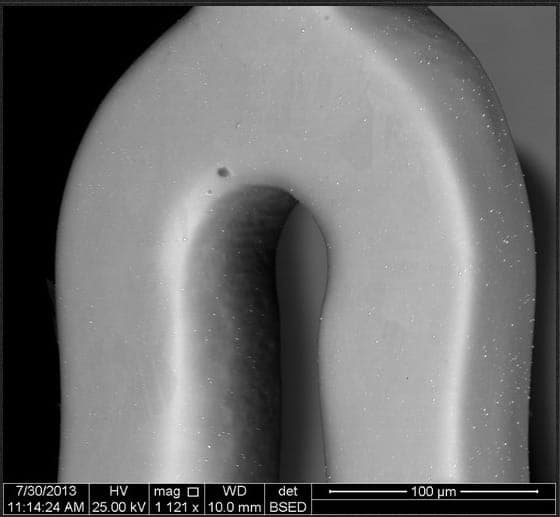


Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
Q. In electropolishing process after completing the EP process on (CoCr coronary stent) we are getting defects on the stent like oxide, pits, etc. Excluding the suggestion of varying the parameter (i.e., current & time), can you kindly help me regarding these defects?
Manisha Kumariemployee - gandhinagar,gujarat, India.
July 21, 2017
A. Hi Manisha. A solution which does not involve changing the current and time may or may not be possible. But please start by telling us the temperature and time that you are employing, and the solution that you are using. Thanks.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
July 2017
A. Dental style Chrome Cobalt alloys can be, and have been, electropolished for over 70 years.
Pre-Made solutions can be obtained from ESMA Chemicals, Nobilium.com, Dentsply.com (Sirona Division), Dentaruim.com. For small quantities, try ESMAINC.COM Chemicals (about US $70 per gallon plus shipping). As well as making them yourself.
60% Ethylene Glycol
40% Phosphoric Acid (85%)
6 amps/sq ft DC
WDR Scientific - Central Point, Oregon USA
August 26, 2017
![]() Thanks Bruce, but can you double check your units? I don't think you mean 6 Amps/sq ft; to me that sounds low by a factor of more than 10 to 1. Thanks.
Thanks Bruce, but can you double check your units? I don't think you mean 6 Amps/sq ft; to me that sounds low by a factor of more than 10 to 1. Thanks.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Co-Cr stents electropolishing is different from other parts electropolishing. Usually if electropolished by standard solution, the outer stents surface is electropolished well. However the inner surface is not polished property.
adv.
We have developed the unique technological process that electropolishes the inner and the outer surface equally on tiny cobalt stents. See image below:
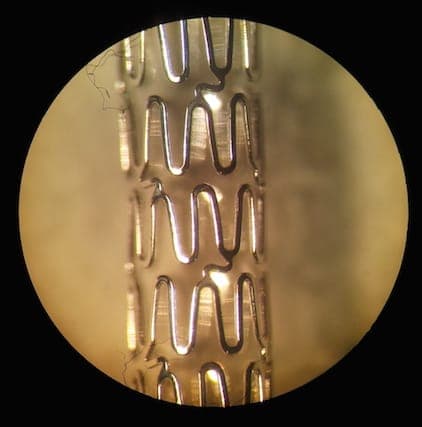
Also our electropolishing is not using hazardous ethylene glycol. We also develop special holders for each PARTICULAR stent design. We can do outsourcing or deliver processing and equipment.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
Q. Which solution would be very effective in electropolishing process of cobalt chromium stent for achieving the inner as well as the outer surface of high quality?
brijesh kori- Surat,Gujarat
January 23, 2018
A. Hi Brijesh. Anna seems to be claiming that achieving good internal polishing without internal anodes will require proprietary solutions. Are you using internal anodes to get some electropolishing current to that area? If so, please describe what those anodes look like.
But several mixes have already been suggested, have you tried any of them like those described by Bruce Dean and achieved a satisfactory finish on the outside, but not on the inside? Is the inside etched, or shows no effect, or what? What solution & current density have you been using? Are you being very careful that water is not being dragged in? Thanks!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
My situation: Is it possible that cobalt chromium stents can be descaled within 1-1/2 hours?
Brijesh Kori [returning]employee - India
July 2, 2018
A. Removing oxides from cobalt-chrome laser-cut stents is a very important step prior to electropolishing. These oxides are more like carbides of various chemical composition. Traditional oxide-removal methods:
a) mechanical blasting - can leave oxide spots that will produce areas that cannot be electropolished;
b) alkali- or acid-based chemical etching - damage the original metal surface underneath;
c) high-voltage plasma electropolishing works fine, however coronary stents are very delicate, and plasma eats up the stent in seconds.
adv.
Our electrochemical proprietary processing completely eliminates oxides without impacting the stent's surface.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
July 3, 2018
Q. Query regarding Carbide layers on the Surface of L605.
Hello, kindly tell me please, after electropolishing the residual carbide layer or simply we can say carbide granules are harmful for the implant or it may causes any reaction in body? If it's harmful, how we can remove these carbides from CoCr L605 surface. Thank you.
Student - islamabad Pakistan
October 25, 2019
Q. Hi Bruce, is it necessary to add water to the acid to have better electrical conduction or does the acid alone conduct well? If necessary, what % of water would be needed in this formula? Thank you very much.
Ricardo Lorenzi- Ribeirao Preto SP , Brasil
September 24, 2023
A. Hi Ricardo,
Bruce's posting was from 6 years ago so he may not be reachable, but Bruce's numbers total 100% without any water, and water is rarely added to any electropolishing solution.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Ricardo,
Just so you're aware, acids are almost always used in an aqueous solution, meaning that water is already there. (Very pure acids that are not a dry powder form are generally extra hazardous or otherwise more difficult to work with, and therefore are most often instead handled in a high-concentration solution with water.)
Also, water itself does not conduct electricity. Extremely low conductivity is one of the common tests for very pure water. Rather, it's the ions dissolved in water that allow it to conduct electricity. Ions, such as... acid. So if you have an acid solution where the conductivity is lower than you want, you should add... more acid!

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

![]() Hi Ted and Ray, I did a test with 15% sulfuric acid and 85% ethylene glycol and got a good result. I used a 12 volt, 3 amp source. I am a prosthetist and my intention is to pre-polish my cobalt chrome prostheses. I will do more tests by adding hydrochloric acid and a formula with phosphoric acid, also provided by Bruce. My aim is to give the structure a uniform shine. The final polishing takes place with abrasives, rubber and polishing paste
Hi Ted and Ray, I did a test with 15% sulfuric acid and 85% ethylene glycol and got a good result. I used a 12 volt, 3 amp source. I am a prosthetist and my intention is to pre-polish my cobalt chrome prostheses. I will do more tests by adding hydrochloric acid and a formula with phosphoric acid, also provided by Bruce. My aim is to give the structure a uniform shine. The final polishing takes place with abrasives, rubber and polishing paste
- Ribeirao Preto SP , Brasil
Q. Hello Bruce. The friend asks ethylene glycol "EF 200". Is it monoethylene glycol? Or is it another glycol? EF 200 means what? It's another type of glycol, I had this doubt. Thank you very much.
Ricardo Lorenzi [returning]- Ribeirao Preto SP Brasil
February 4, 2024
A. Hi Ricardo.
Bruce doesn't seem responsive to postings here or e-mail to the address we have anymore, but I would suspect that "EF 200" is just a brand name.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Electropolishing white patch issue
Q. We are getting White patches after electropolishing cobalt chromium material.
We suspect it's because the EP solution not wiping out properly even after cleaning in ultrasonication, that causes the white patch after passivation.
How can we overcome this
- Mysore Karnataka
July 6, 2024
A. Those are carbides that do not dissolve in regular CoCr electrolyte. Our way to overcome them in CoCr hip implants is with a final touch of brief electropolishing in plasma.
As for CoCr stents - it is more complicated. Prior to electropolishing the delicate coronary stents need to be gently treated by softening and removing laser oxides. And only then a very brief electropolishing in electrolyte that does not leave those hard carbides on the surface.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
July 10, 2024
Q, A, or Comment on THIS thread -or- Start a NEW Thread
