
-----
High dielectric yet high conductivity!?
Tell Me It Ain't So. I have a customer asking us for a coating for aluminum to replace a proprietary finish they're now receiving in which the competitor list the coating as "offering high dielectric strength yet highly conductive." Is this even possible?
Milt StevensonPlating Shop Employee - Syracuse, New York
2007
It sounds possible to me. I understand a dielectric to not be merely a good insulator, but one that allows free flow of magnetic forces. If you were able to successfully electroless nickel plate an anodized surface you would have a plating that is quite conductive but the plating would be well insulated from the aluminum substrate and would (I think) give you good dielectric properties between the nickel and the aluminum.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2007
Can't you just ask your customer to clearly define what he wants instead of trying to get puzzle solving help in the net?
Guillermo MarrufoMonterrey, NL, Mexico
2007
January 16, 2008
1. We have high respect with this website. And we recommend this site to our professional friends.
2. With all due respect, the statement that the material or finish exhibits DIELECTRIC AS WELL AS HIGH CONDUCTIVITY is out of line, it confuses a lot of people around me.
3. We are not chemists here, but we are involve in the semiconductor business. We know DIELECTRIC is synonymous to insulation (meaning it does not pass current), and , high CONDUCTIVITY means it can easily pass current. It does not follow the laws of PHYSICS or are we missing a point?
Hope to qualify the statements made.
With respect,
Joseph
- Imus, Cavite, Philippines
January 16, 2008
Thanks for the kind words. Certainly a single homogeneous material cannot be both an insulator and a conductor. But a multilayer coating system could certainly have both a highly conductive outer layer and an insulating layer which electrically isolates it from the substrate per attached sketch.
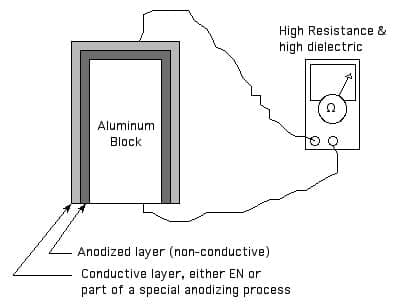

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
I think that you definitely need to have a talk with the customer to find out what he really wants. I am not willing to bet anything that costs much or needs to be foolproof on having a plated coat over anodize never make contact. Next. how much current does it need to carry. Nickel is a relatively poor conductor. To me, dielectric means very little current flow and that is relative to the need. 1 milliamp can be a disaster on an electronic device and 1 amp would not be a problem grounding a housing, but might not be enough.
James Watts- Navarre, Florida
January 17, 2008
Milt, could he be thinking about an anodic coating with the pores filled with tin, silver, or copper? There would be a slight "dielectric film" in between, the aluminum metal and the conductive top coat. Years ago "Metal Finishing" ran a research paper on a process where the anodizing solution was loaded with copper, then varying degrees of reverse current were used as he combined anodizing with copper plating, at first more anodic and less cathodic, then as the process went on he gradually changed over to less anodic and more cathodic.. Then you have the so-called two-step tin process. Then one brand name company that was visting your plant the last time I was there, has been trying to sell a procedss for putting silver into the pores for top side "conductivity". Way Out !

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

January 18, 2008
Is the "Al-anodize Al-copper" two step process is successful? So the anodize layer become an electrical isulator later for the copper (or electrical insulator for the aluminum)? Is this possible? I try it before but not the way you mentioned above (slow changes). What I do is hoping to form electroless copper on top of the anodize surface then start a electrolytic plating on the electroless layer, Although I manage to plate the electrolytic copper, but later found out actually I lost the anodize layer during the electroless copper process. If I use electroless Ni, am I going to face the same scenario?
Ron Ng Ah HockPlating Shop (services & hobby) - Bagan Serai, Perak, Malaysia
February 17, 2008
March 3, 2008
Mr. Milt Stevenson,
Is there any update to what happened to your customer's High Dielectric Yet High Conductivity Coating?
Thanks in advance
Regards
Joseph FRanco
Mr. Ted Mooney,
Thank you for your reply, but I really still don't understand, really sorry for this. An OHMMeter measures resistance in OHMS, and for highly conductive this is very close to "ZERO OHMS". But if we use it to measure insulation HIGH DIELECTRIC then reading or needle will be somewhere opposite ("ZERO OHMS")on the other side of the scale of the OHMMeter. So you see it really is confusing.
I believe you are right if what you are trying to say is that it is some kind of DIODE in action. In Diodes, CONDUCTIVITY and RESISTIVITY do happen that is why it is called semiconductor, but true only if we reverse polarity of the OHMmeter or DC Power source.
Could it be that the coated component is some kind of High Performance Power Diode? WILD GUESS.... But I'd love to own one,tho. Hope Milt Stevenson reads this.
Thank You,
Joseph Franco
- Imus Cavite, Philippines
Q, A, or Comment on THIS thread -or- Start a NEW Thread