
-----
Chromate conversion on cadmium not adhering
Q. We have a steel stamping that is cad plated with chromate conversion- it is then painted.
We have problems with the paint coming off and it appears to take the yellow conversion coating with it. Any ideas what could be causing this? It appears the conversion is not adhering to the base cad metal.
machine shop - Falconer, New York
2007
A. I think you'll need to make contact with the plating shop and have them help figure out what's wrong, because:
• It could be just excessive brightener or allowing two thick chromate, and the next batch could be just fine; but
• It could be a move to a trivalent chromating which meets salt spray requirements but introduces new adhesion issues, and perhaps the problem won't go away until they make a major change.
Unfortunately there's a lot of possibilities :-)

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
|
A. I've seen problems like this arise both from excessive brightener, as Ted suggests, and from poor post-plate rinsing.  Dave Wichern Consultant - The Bronx, New York A. Joe - Falconer isn't all the far from Syracuse where my shop is located. It would be a whole lot easier to see the actual hardware that is exhibiting the problem (if at all possible before and after painting). I'd be willing to do so if you're willing to send in a couple of parts to review. People like to point their finger at the plater, but contamination post-plating is a likely cause as well. Have you gotten good parts in the past? Have you changed painting houses? How long was the lag between plating and painting? Do the parts require embrittlement baking between plating and chromating? There's a lot more to solving this issue than "why is the paint coming off?"  Milt Stevenson, Jr. Plating shop technical manager - Syracuse, New York A. I think there is no need to chromate at all if a topcoat of powder or paint is being used. Cadmium is used because it forms its own oxide topcoat which retards further corrosion, unlike zinc that corrodes away.  Khozem Vahaanwala Saify Ind Bengaluru, Karnataka, India  |
Q. The passivation done after cadmium plating is coming off on simply rubbing with cloth.
Can somebody please suggest remedial measures?
Thanks & Regards
- kanpur, India
June 13, 2017
A. Hello Sahil!
Usually if you can remove the chromate with your fingers or a cloth, there are two big possibilities:
- Organics in your cadmium/zinc bath are too high.
- Concentration of the chromate bath is too high.
This two possibilities have a relative probability that depends on each facility and methods, so please explore them and let us know what you find!
Best regards!
TEL - N FERRARIS - Cañuelas, Buenos Aires, Argentina
Q. Hello all,
I have a small cad plating tank for doing small parts. Everything was working very well until I lost my finish gold dip bucket; it cracked and leaked out.
After getting more gold dip and mixing according to the recommended ratios, same as before, I cannot get the gold to stick.
It will coat evenly but wash off.
The cad seems to be coming out nice and bright; just cannot get the gold dip to adhere.
- Memphis, Tennessee USA
November 26, 2017
by World Health Organization

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Chip. I am guessing that when you talk about 'gold dip' you are referring to a yellow or gold colored chromate conversion coating designed for cadmium plating. So we appended your inquiry to a thread about that problem.
Daniel has given you two good possibilities, and your statement that "the cad seems to be coming out nice and bright" might be a clue that you have too much brightener (organics). But it is not quite clear to me whether you are attempting to chromate conversion coat freshly plated parts and it no longer works, or whether the problem might be that after you got a replacement container and replacement solution and made up the process, you are now attempting to chromate conversion coat cadmium plated parts which you plated some time ago (before the leak); if that is the case, the surface may no longer be active enough for reliable chromating anymore. One other thing you may realize but we'll say for the benefit of newbies who (You didn't mention catching the leaked solution in a secondary containment vessel; I hope you have researched the toxicity of cadmium and hexavalent chromium; they're bad ones. A bucket of chromate solution can do quite a bit of environmental damage, and you should definitely have secondary containment).
Luck and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Need cadmium plated yellow chromated screws black chromated
April 30, 2018Q. I currently have 100 ea #10-24, 1/2" long screws (MS16997-44) that are cad plated to spec with a yellow chromate finish. I need them to be finished in a black chromate. I failed at using a hobby plating chromate. I have conceded and will now be going to a plating shop for help, but am looking at feasibility and an explanation to what might have happened right now.
Question 1: Can the black chromate be applied on top of the yellow chromate (this is what I tried?) It looks like the hobby plating system is designed to be used on zinc or cad substitute (not real cad).
Question 2: Can the yellow chromate be removed /replaced with the black chromate without harming the cadmium plating (not asking for DIY.)
I tried to use the hobby plating black chromate according to directions and it was not a uniform black coating. Eventually the parts went from black to grey and I assume the cadmium and chromate dissolved and I was left with a bare steel parts.
Buyer - Selmer, Tennessee USA
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. Hi Larry,
I have used a 10% caustic solution to remove the yellow chromate from cad in the past when I needed the parts dyed green, blue or red, so I'd guess that this approach should work for black dyeing. The one thing you can't do is try to apply the dye over the already chromated part, it just doesn't work.
Aerospace - Yeovil, Somerset, UK
May 2, 2018
Discolored yellow chromate on cadmium plating
Q. First of all, I am a layman to industrial plating. However, I am really keen to solve the problem I am having now.
Problem:
Recently, my customer complained to us that the connectors they received from us found discoloration of yellow chromate. (Our company does not produce the connector, we assemble wire on it) The connector is finish in SAE AMSQQP416 Cadmium Plating. I did not find any standard for chromate treatment such as thickness, adhesion, corrosion resistance, etc.
Question:
What is the possible root cause of the discoloration of yellow chromate, or it is normal?
Quality Engineer - Kuala Lumpur, Malaysia
November 14, 2018
A. The plater did not neutralize off the cyanide before entering the yellow chromate.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

November 14, 2018
Q. Hi Sir, I uploaded some photos, could you please look into it? There are some large scale of yellow chromate peeling off from the surface.

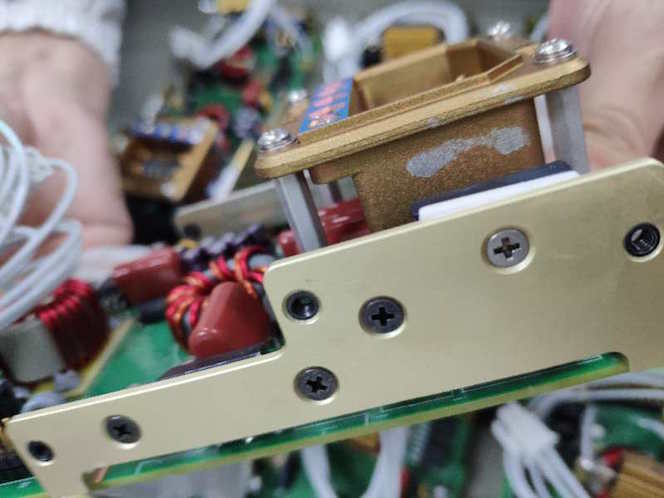
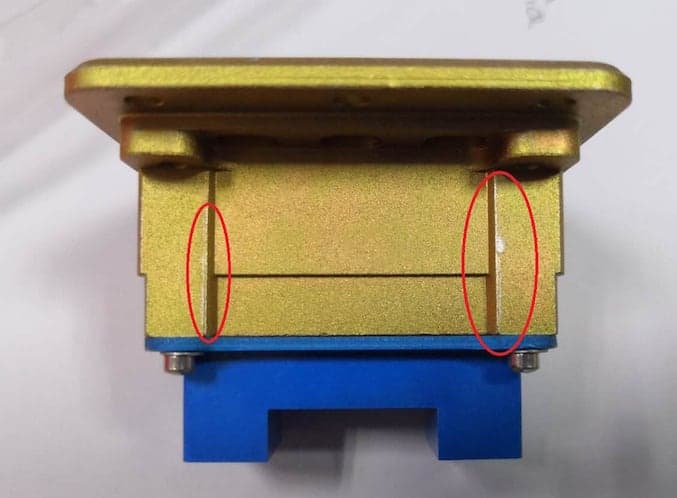
It is the same as what you mentioned?
Leong Corden [returning]- Kuala Lumpur, Malaysia
November 16, 2018
by Larry Durney

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Leong. There's a good chance that Mr. Probert is right. But there are other possible causes including the chromate being dried at high temperature (above 140 °F / 50 °C) before it has fully cured.
The components are not satisfactory in this condition, but figuring out what an unknown plating shop is doing wrong without benefit of walking the line, chemical analyses, examining the records, or holding the actual parts is problematical.
Your postings and effort at learning are very welcome, and I'm not trying to discourage you from it ... I'm just forewarning that trying to solve plating problems that way is hard and may be wasted effort. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 23, 2018
After discussion with our customer we found in their final process after the connector assembled on PCBA it will be sent to cleaning.
Cleaning Process Parameters:
Cleaning Fluid: DI Water
Fluid Temperature: 150 °F
Spray Pressure: 75 PSI
Spray duration: 12 Minutes
Does the above process cause damage to the surface? And why?
Quality Engineer - Kuala Lumpur, Malaysia
A. Hi again Leong. I am not quite following ...
Are you saying that as long as the components are not cleaned the chromate discoloration does not occur? If that is true, that's helpful data! The large splotch of missing chromate does in fact look like poorly adhered chromate that was pressure washed off, and pressure washing a poor coating can certainly partially strip it.
But if you're saying there is no data available about whether the discoloration occurs before or after that final cleaning, guessing about whether the final cleaning is the cause probably isn't helpful :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 2018
Q. Hi Mr.Ted,
Sorry for the incomplete information.
For your question:
1. If you see above 2 pictures large area of chromate peel off. This is happening all after this cleaning process.
2. Bottom 1 picture chromate peel off at the edge, it happens before the cleaning process, the yield rate is 1.37% (5 of 364 pieces). But all happen at the corner or edge area.
Can you guess the above situation?
Quality Engineer - Kuala Lumpur, Malaysia
November 26, 2018
A. Hi Leong. You must solve the defects that arise before the post-cleaning process before you worry about whether the post-cleaning process causes additional defects. Yes, the pressure washing is apparently knocking some of the chromate off, but until you are producing good parts that have sufficient reliability & adhesion that the parts do not spontaneously fail, it's just a waste of time to speculate about whether the post-cleaning is making it worse.
Have you been able to find out from the plating shop about neutralizing the cyanide before chromating, and about the drying temperature? Thanks! And good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 2018
Q. Dear Sir;
I am Irem from Turkey. I work for the aviation and defense industries.
We did Cd plating in accordance with AMSQQP416 Type-2 Class-3. The coating operation had been done with immersion application and CN content.
The parts are cylindrical but the diameter is not enough to use leakage anode.
In addition, there is a tier, whose height is almost 15-20 mm at the end of the cylinder. This is the shape of the parts and at this tier dark view.
Why did the dark area occur at the tier?




The dark area is seen in the tier of the parts. By the way, parts have painted the outer surface. In addition, the parts have a machining operation. There is no welding area.
Thank you very much for support.

Irem Tuncel Boğaz
Chemical Coating Engineer - Izmir, Turkey
A. Hi Irem. Please try to send better pics; unfortunately, those are are not very good :-(
At first I thought you were concerned about all those dark red blotches and dots until I noticed that they are everywhere, not just on the parts. But it does look like you have some areas with no cadmium plating coverage. My guess is that this is not a problem with the geometry, but something about the processing in that flared area, or perhaps a skim of oil on your cleaning tank that catches in the flared area. After your usual cleaning, please try to scrub that area very well with a scrub brush ⇦ on eBay or Amazon [affil link] and pumice ⇦ on eBay or Amazon [affil link] & water, then rinse and proceed to your acid activation step and tell us / show us the result. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 2019
Q. Dear sir,
We are doing Cyanide based Cadmium plating 9 micron on steel parts. The operation sequence is: Degreasing in organic solvent- Derusting in dilute hydrochloric acid- Cadmium plating in barrel - water rinse- Nitric acid 0.5-1% activation- Chromate passivation in proprietary hexavalent chromate bath- water rinse- Air dry.
After complete process, the chromate passivation found not adhered & removed by simple rubbing/handling.
We are maintaining the cadmium plating bath by keeping concentration as: cadmium content (14-35 gm/lit), NaCN (65-100 gm/lit) & NaOH content (20-40 gm/lit). The chromate passivation bath operated at 6-8 ml/lit at Room temperature.
Kindly suggest the remedy for improvement in adhesion of chromate passivation.
- Nagpur India
February 4, 2021
A. Hello Pankaj,
Once cadmium has been chromate passivated the surface remains fragile for up to 24 hrs after dipping. The coating needs time to stablise before it can be handled. We give our cadmium plating a 24 hr hold once done before handling and or painting etc. AMSQQP416 details this, as do other specs.
- A covid free rock, (for the 2nd time) in the Irish sea.
Q. Hi Mark Lees.
Thanks for response.
We know that chromate passivated parts are not to be handled up to 24 hrs.
However, our problem of discoloration occurred after 24 hrs. By simply rubbing the parts by fingers, the chromate layer is easily removed.
We have taken the trials by reducing the chromate bath concentration, Bath dilution & filtration, but still no satisfactory results.
Kindly suggest.
- Nagpur India
A. Hi Pankaj.
It sounds like Hull Cell
⇦ huh?
panel testing might help you see if the chromate adheres.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Aloha,
Did you check your pH on the rinses after chromate?
the pH of the rinse affects by two ways:
1)pH acid, chromate coming off.
2)pH alkaline, Color tends to be dull and with discoloration.
- Nogales, Sonora
March 23, 2023
Q, A, or Comment on THIS thread -or- Start a NEW Thread
