
-----
Trivalent and hexavalent chrome analysis
Q. Is there equipment that allows for monitoring the concentration of chromium trioxide in the chromium plating bath in real-time, i.e., a sensor immersed in the electrolyte that outputs data to some terminal or something like that?
Nik Erm- Nizhniy Novgorod, Russia
December 18, 2024
A. Nik, there is a company that makes a hexavalent chromium active analyzer, but I don't see that they offer the same equipment for trivalent. The company is Hach. I cannot speak to the efficacy or product quality, only that the sensor does exist. They also do not list the prices online.
I know this is only a partial answer, but I hope it helps you find the right direction to look.
Lab Rat - Fort Wayne, Indiana
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. I would like to know if there is a titrimetric procedure of analysis in determining hexavalent and trivalent chromates. Can you suggest where I can get them?
by Langford & Parker

on Amazon
or AbeBooks
(affil link)
Researcher - Philippines
2007
A. Most books on Electroplating Analysis have the method, e.g., Langford's "Analysis of Electroplating and Related Solutions" [on
AbeBooks or
eBay or
Amazon affil links]
, the Electroplating Engineering Handbook ⇦ this on
eBay,
AbeBooks, or
Amazon [affil link]
, etc.
Hexavalent is by iodometric titration with sodium thiosulphate
⇦ on
eBay
or
Amazon [affil link]
.
Trivalent is by oxidizing the trivalent with peroxide in alkaline conditions, boiling to destroy excess peroxide and then acidifying and doing the iodometric titration above and the trivalent is determined by difference.

Geoffrey Whitelaw
- Port Melbourne, Australia
Q. Is there any simple method that can accurately determine the concentration of Cr6+ and Cr3+ in plating solution? Will Ion-chromatography do the job? Thanks for your help in advance.
Tommy YuenPlating engineer - China
October 12, 2009
Q. Hello, We are doing hard chrome plating (among other platings), well we are having some confusion in the determination of trivalent chromium, we checked some written procedures, and we are confused about how they consider this trivalent chromium, is Cr3+ or Cr2O3)?? In this bath we are controlling ranges of chromic acid,ratio chromic acid sulphate, trivalent chrome, Iron, Temperature.
Well I hope you could help me; thank you very much.
Engineer - Peru
September 13, 2011
Q. My name is Peter Totaro and I am a chemist for a company in Paterson, NJ. I'm also in my last year of my Masters in Chem Engineering. I have a question for you. I would like to know if there is a way to calculate trivalent chromium in a sodium dichromate/nitric acid passivation bath. I need to prove out a density calculation by hand and I'm having trouble determining the trivalent chromium. Is there anyway this is possible?
Thank you,
- Paterson, New Jersey
December 30, 2014
A. You can determine total chrome and hex chrome. The difference is tri chrome.

James Totter, CEF
- Tallahassee, Florida
January 7, 2015
A. We analyze our hexavalent chromate solutions for Metal, sulphate, and Fluoride.
Metal analysis has Na2S2O3 as the titrator, sulphate analysis uses Kocour A & B w/centrifugation, and Fluoride slopes are determined by TISAB III, 20% C2H3NaO2 and 1000 ppm Fluoride standard.
The results determine any additions or dilutions needed.
OEM decorative chrome plating - milwaukee, Wisconsin usa
January 24, 2015
Solution Analysis of Immersion bath in Chromate Conversion Coating Process
Q. My situation:
We are a Aerospace Component manufacturing shop. We have recently introduced a chromate conversion coating process in house. Our customer requirement states that we have to perform solution analysis of the Immersion bath periodically. Is there a standard laboratory process to do that. I have found couple of spectrometers from Hach. Are there any other gages or controls that I can setup to monitor the concentration of the Chromium bath? Any Sampling Procedure?
Thanks in Advance
Engineer - Traverse City, Michigan, USA
July 25, 2018
A. Hello Mr. Achyuta,
Please give the manufacturer and exact chemical name that you are using. Usually, you can find control instructions on their tech data sheet, and with some practice and a couple tricks, get a good result.
The lab analysis for a RoHS-compliant conversion bath is more difficult and requires more equipment, but for traditional hexavalent chromium products, a simple oxidation-reduction titration will give you good data. Let me know, and I'll get back to you with the best information I have!

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Q. Thanks for the reply Rachel.
The product that we are intending to use is Bonderite M-CR 600RTU (Henkel Product).
Also some follow-up questions
1.) Since this is an immersion process (not plating just chromate conversion) how can I monitor the contamination in the chromate bath?
2.) Is there a documented procedure for that?
3.) Will a total chrome test on the free chromic acid do the job?
Thanks in advance for considering.
- Traverse City, Michigan
A. Hi there, I see that the Tech Sheet DOES have a solution analysis section, but it appears to require ready-mixed reagents that are not identified as to composition or concentration:
http://www.solvents.net.au/index_htm_files/ALODINE%20600%20(169313).pdf
That being said, you should be able to set up a standard calibration curve and run it through Excel and get your multipliers. It will take extra precision and care setting it up, but give you valid results, and you only need to generate the curve once. You'll want to document the heck outta the process of generating your curve.
This is how I would approach this, if I didn't want to buy their pre-mixed stuff:
-Use only pure deionized water when making your standards and dilutions
-Use a calibrated analytical balance that weighs to 3 digits
-Use class A volumetric flasks for your dilutions
-Test your pipets using DI water and your balance... and write down the results!
-DOCUMENT EVERY STEP OF THIS PROCESS because an auditor will ask where you came up with your multipliers!
Set up your standards as a blank (just the DI water you are using), a standard that is about half of your operating concentration, a standard that is at or near your operating concentration, and one that is twice your concentration. Don't be tempted to do serial dilutions (preparing a standard from a more concentrated standard), as that will just pass any imprecisions along! Also, it's a great idea to make each standard in duplicates so that if you run two of the same, and they don't agree, you know that you had an error.
Okay. This is a pretty basic oxidation-reduction titration. I've seen it done with permanganate, but I hate that stuff, and prefer the iodometric method.
You need:
Sodium thiosulphate 0.1N
Sulfuric acid 50% (this does not have to be exact, it just acts as a conditioning agent for the sample, but it DOES have to be pure)
Starch solution in water. 0.5% - 1% is fine. Again, does not have to be exact. It will often be preserved with salicylic acid, don't worry about that, as it does not affect the outcome
Potassium iodide solid
a 5mL pipet that you have proven to be accurate
Load up a 50mL buret with Sodium thiosulphate and zero it.
Set up a stir plate. You really need one; the iodine goes in as a solid and takes a little while to dissolve, and also the endpoint is too twitchy to hit while swirling and dripping and watching all at once.
Using the pipet, put 5mL of Sulfuric acid and 10mL of your standard or sample in a 250mL beaker [beakers on
eBay
or
Amazon [affil link] . Add about 50mL DI water. Doesn't have to be exact, but the stir bar needs to spin freely and you want enough liquid to dissolve the KI.
Add about 2g Potassium iodide solid. Does not need to be exact. The solution will turn very dark brown and temporarily cloudy. When the cloudiness is dissipated and the solid chunks are gone, you can start titrating.
Gradually add Sodium thiosulphate out of your buret. The solution will start to lighten. Don't take TOO long to do this, as the reaction is sensitive to atmospheric oxygen.
The solution will eventually reach a pale straw color, possibly with a greenish hue. Stop titrating and quickly add about 2mL starch indicator. The solution will turn inky blue-black. NOTE: IF THE SOLUTION DOES NOT TURN BLACK, YOU WENT TOO FAR. START OVER, AND ADD THE INDICATOR A LITTLE EARLIER. Start titrating again but this time more slowly, a drop at a time. Not TOO slow, but you now need to watch like a hawk because the endpoint comes up fast. There will be a point where the addition of a single drop will turn the solution very suddenly clear, with a very pale color that can range from straw to blue. But CLEAR.
Write down your mL titrated. If you needed more than 50mL, stop titrating at exactly 50.0 and re-zero, and just add the two values.
Repeat with all standards and a blank, and use this to set up your curve.
If you have multiple technicians, test them all to ensure that their technique gives the same results- this titration does have a bit of an art to it. Adding the starch too early and also titration speed can somewhat skew the results. Also, you may notice that after the solution clears, if you leave it on the stir plate it will cloud up and blacken again. That is the atmospheric interference. Don't second-guess your initial result!
Okay so for the rest of your questions:
-Just testing for Chromium isn't going to give you as accurate a picture of bath health as the titration described above.
-As you noted, contamination DOES play into it, and things you might consider testing for are sulphate, dissolved Aluminum, and Silicon. Now, that being said, the formulation as-bought will have baseline levels that you should establish by sending your as-mixed-in-tank level standard out for testing at a lab and then periodically (depending on throughput) sending out bath samples to make sure nothing has spiked. Conversely, substances that you may NOT be testing for, added to the material as activators, can be depleted, and a bath that appears to be 'healthy' may not behave as expected. This is a conversation to have with your sales rep who can tell you what other users test for. I use Iridite 14-2, and I test for sulphate buildup. Your mileage may vary ;)
sulphate is typically run by EPA 300 (ion chromatography) and metals are run by EPA 200.7 (plasma spectrophotometry). Save yourself a hundred thousand bucks and a Volkswagen sized chunk of floor space, and outsource that mess!
Once you have your analysis method down, you also will want to set a schedule for testing. You can set your own schedule, but you have to stick to it, and may be asked to justify your interval to an auditor. If you find your tank goes out of spec between tests, you have to test more often. Auditors will torch you on that!
Good luck- if you have any issues, come on back :)

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Q. Thanks a lot for all the useful information Rachel. I was able to find a lab to outsource this and have the results with me.
I had the lab test for total chrome, conductivity, metals (iron) and sulphate. Is there anything else I am missing in order to determine the contamination in my chromium bath??
What are the permissible limits for each item? I know I can determine the base line by testing the new sample but just wanted to know the Min-Max permissible levels
Thanks in advance
- Traverse City, Michigan
August 20, 2018
A. You're going to have to talk to your distributor's technical specialist about that; I only run Iridite 14-2 and my levels will not likely be the same as yours.
If you have a set schedule for contamination testing in both your Alodine tank and your DeOx tank (test deox for Si buildup and keep it safely under 30 ppm- if you run castings it will rise faster), you can trend your contaminants data for both tanks against your results from salt spray testing and see where you start to have a couple pits and compare that to your contaminants data and set your own acceptable levels for how high things can get before the tanks start misbehaving. I say 'tanks', because those two tanks are a SYSTEM that work together.
Remember that for any experiment to be valid (and this is indeed essentially gathering experimental data), you have to be able to isolate your variables which means keeping the Alodine levels, pH, rinse tank conditions, etc steady over time, and then analyzing your results carefully. Talk to your tech rep! :)
Another thing I feel I should reiterate:
Testing for total chrome may not give you accurate results in terms of, 'how well can I expect my bath to work?' for a couple of reasons. One, that bath's success depends on how 'active' the chrome in it is, which may not have a perfect correlation to total chrome, and you need to monitor your oxidation-reduction potential to do that. Two, aging conversion baths can end up with trivalent chromium sloshing around and doing nothing productive- like a worker who is technically clocked in, but taking an indefinite smoke break- both types will still show up in a 'total chromium' analysis. Iridite 14-2 actually has a 'bath life' test that involves raising the pH of a tank sample to precipitate out the tri (and some other metallic contaminants), and looking at how much crud ends up in the bottom of a graduated cylinder. It's crude (and I find it gives a false sense of security), but the fact it was included on the tech sheet... well, there's a reason.
Likewise, conductivity testing isn't going to tell you a lot. It will include bath components but also contaminants. Like counting a jar of marbles in the dark but you are only looking for red ones and can't tell the difference by feel. Conductivity testing is more appropriate to rinse waters, in which case EVERYTHING in there matters.
Sorry my analogies are so groan-worthy, it's Monday and that's all I can muster whilst trying to reclaim my desk from the maelstrom that apparently came through over the weekend.

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Q. Hello everyone,
I'm now doing a research on how to measure the concentration of the compositions (H3CrO4, H2SO4, Cr3+) in chromium plating, online.
We prefer to do this though optical methods,such as absorption coefficient.
Since the Cr3+ and Cr6+ have different color, we can differentiate these two easily. But, because the concentration of Chromic acid is too high, so there will generate Dichromic acid, they have similar color, it's very difficult to distinguish these two.
My question is :
1. Is there any method that I can know the ratio of these two acids?
2. Can Anhydrous sodium sulfite treat Cr6+ to Cr3+ ?
Thank you very much.
- Hong Kong, China
December 6, 2018
A2. Hi Bree,
Sodium metabisufite is the most common solid chemical used for reducing Cr+6; Sodium sulfite would theoretically work too, but you'd need more of it to treat an equivalent volume of chromium solution.

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
A1. Hi Bree. You can analyze for Cr+6 but I'm not sure that it's even possible to analytically distinguish CrO4-2 from Cr2O7-2 while in solution, and they ionize to HCrO4-1 as well. The Wikipedia article is a bit beyond my chemistry skills but indicates that the three will morph dependent on pH and concentration.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi sir,
I am Working in lab chemist in lab chemist surface finishing process. Cadmium plating process, metal is using mild steel, colour is chrome passivation bath preparation is using Sodium dichromate and sulfuric acid. Aero space industry is following the method ...
Chrome passivation titration procedures. I prepared in bath 1) sodium dichromate in 20g/litre 2) sulfuric acid 6ml/litre contractions how identified. Thank you sir.
With regards
Taneja aero space & aviation Hosur - Hosur, Tamil Nadu , India
October 14, 2019
A. Hi Sivakumar,
If I am understanding correctly, you want to learn how to tell the concentration of chromium in your passivation bath?
Use the method described above, and then record mL Thiosulphate from your titration as “A”.
( 0.664 A ) / 5 = oz/gal Sodium DiChromate
.1328 B = oz/gal sodium dichromate
(( 0.664 A ) / 5) x 7.489 = g/L Sodium DiChromate

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Calibrate a spectrophotometer with home-made standards?
Q. A couple of days ago I bought a visible spectrophotometer really cheap around 300 USD and I need to calibrate it. Does somebody know how I can do it (indicating to me a book or something)?
I saw on the internet that some standards are available to calibrate it, but they are very expensive and I can't afford it. I want to analyze a chromate conversion bath and I was thinking maybe I can prepare 3 solutions with different concentrations of the bath to prove the linearity of the spectrophotometer? Could this be a good idea to corroborate the spectrophotometer?
- agua prieta, Mexico
September 10, 2020
A. Hi Aaron!
Your standards come in 55 gallon drums; all you have to do is make them! The good part is, this is easy. The bad part is, you should calibrate your spectrophotometer every single time you use it due to bulb aging and variations in temperature and on-time for the instrument.
I assume this is RoHS chromate since yellow chromate is tested via oxidation-reduction titration?
This is how you can check a TCP-HF bath:
Warm up the spectrophotometer and set it to ABS [absorbance] mode at 600 nm. If it has a 'scan' function, you can confirm your max-absorbance point on a standard and fine-tune to that wavelength. Use Quartz cuvettes, preferably.
Using Class A Volumetric Flasks, deionized water, and calibrated pipette [pipettes on eBay or Amazon [affil link] , prepare the following calibration standards from concentrated TCP-HF stock solution:
10%vv- 10mL in 100mL
20%vv- 10mL in 50mL
50%vv- 5mL in 10mL
Or whatever you like to bracket your target concentration, such as 0, 50%, 100%Target, and 200%.
If you add a 75% and 125% standard, you'll increase your accuracy! However, do not be tempted to do serial dilutions... it adds inaccuracy. So make whatever convenient dilutions you can with the glassware and pipettes you have on hand.
Shake well to mix and then allow to settle if any cloudiness is visible.
Zero out the spectrophotometer on Deionized water.
Put in each test standard, wait for stability, and write down the reading.
Put in your tank sample, repeat.
Using the standard spectrophotometry worksheet which you can make in Excel, enter values for all standards and the settled tank sample. Generate a calibration curve using Excel, and use that to get your values.
Here is a screen capture. Look at the raw excel formula way up top; you can see where the numbers came from after the standards were entered and a function was generated. I can't remember what this data set was for, probably a dye tank lol. Bane of my existence. :)
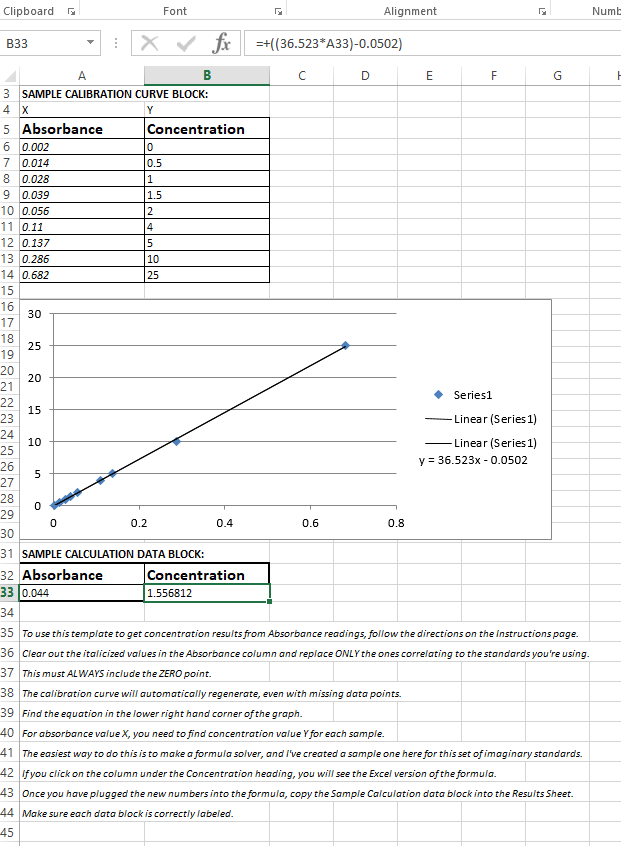
When you're done, just print out your worksheet and keep it with your raw data hoard in case an auditor wants to have a look.

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Lab Rat
![]() Hi Rachel
Hi Rachel
Thanks for your constant support I made it as you said and every works perfectly, you saved me again
- Sonora Mexico
October 14, 2020
![]() Aaron, my pleasure as always ... and I actually get to thank you this time as well for jolting me into Fix My TCP Tank mode! It's behaving perfectly, but I have had an unusual experience over the past couple weeks with a spectrophotometry lab result coming back as "invalidated" due to the tank sample "looking unusually blue" (I mean, welcome to 2020, aren't we all? My 2nd fiscal quarter nachos consumption is a testament to that) and supplanted on the report by a titration result, which is notoriously difficult to nail repeatably from Cr+3.
Aaron, my pleasure as always ... and I actually get to thank you this time as well for jolting me into Fix My TCP Tank mode! It's behaving perfectly, but I have had an unusual experience over the past couple weeks with a spectrophotometry lab result coming back as "invalidated" due to the tank sample "looking unusually blue" (I mean, welcome to 2020, aren't we all? My 2nd fiscal quarter nachos consumption is a testament to that) and supplanted on the report by a titration result, which is notoriously difficult to nail repeatably from Cr+3.
I got the bug in my hat to fire up the c.1994-1996 Probably Mildly Radioactive Bench Brick and run a manual 'scan'. GotoWavelength... beep beep... ABS... write result... repeat ad nauseum... comparing bath vs drum peak absorbance, the peak absorbance drift was only about 10 nanometers on a 5 year old bath. Back in the day (my records date to 2009), when QualiChem produced it, there was actually part of the CoA listing peak absorbance on the batch as a test criterion. I miss the old days! That was a useful number!
So what I would like to add to my previous post, is to find max absorbance for both the tank sample and your 20%vv standard, and choose a wavelength for the actual test halfway between those two points. If you find you've got really significantly different max absorbance wavelengths, you should check with your tech contact, but I was pleasantly surprised to see how well this antique-by-plating-tank-standards bath is holding up.
Anyway... glad all is well with your testing, and best wishes for a stable and predictable tank!

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
October 24, 2020
Q. I was so glad to see the post regarding the calibration of the spectrophotometer! I am looking for a procedure to analyze our chromium plating bath for trivalent chromium. I need a fairly simple procedure for our employees on the manufacturing floor to perform. The titration procedure would be way too complicated and the visual color method would not provide an actual trivalent chromium value. I am looking into the spectrophotometric method and am only finding vague procedures. Does anyone know of a procedure or an instrument that could provide me with a trivalent chromium value? Thank you in advance!
Rebecca Batty- Peru, Illinois
February 17, 2021
A. Back in the days when I ran a lab, I was unhappy with the traditional trivalent procedure for checking my 5 hard chrome baths. It is subject to compound titration error, so you can get very different results from repeat titrations. For that reason, I developed a spectrophotometer method at 600 nm. The essential steps involve, making calibration standards and prepare your calibration curve -- include CrO3 at the nominal bath concentration in all standards, but vary the trivalent in the range that you want to measure. Use DI water as a blank. Dilutions will likely be required to get the standards within the range of your spectrophotometer. The curve really only needs to be developed once, but I chose to revalidate it annually.
The measurement is simple, zero the instrument at 600nm with DI water, dilute the bath sample to match your calibration dilutions, measure absorbance and calculate the result according to the equation from the curve. I hope this helps.
The particular instrument that I used was very basic- It was a Unico 1100. It seems that it is still available on Amazon. In any case, any visible spectrophotometer will work as long as it allows you to develop your own methods instead of forcing you to use their test kits as some brands like Hach seem to do.

Jon Barrows, MSF, EHSSC
Kansas City
Ed. note: Great to see you again, Jon. And the readers always appreciate your knowledgable responses :-)
![]() Jon,
Jon,
Thank you so much for your response! It was extremely helpful.
Q. I do have a question regarding the calibration standards. I am not an actual chemist so I am a little confused at to what Cr I should use for my standards. I have found an old procedure which references using chromium sulphate for making a stock solution and dilute calibration standards from it. Would you agree with this or could you make another suggestion? I have also been looking into purchasing a analytical grade standard to use as a stock solution but once again, not sure what to even look for. Thank you for your help.
Rebecca Batty [returning]- Peru, Illinois
February 23, 2021
A. Hi Rebecca,
If this is actual tri-chromium plating rather than trivalent conversion coating bath where you can easily run the spectrophotometry procedure exactly as I've written below, you're likely going to be stuck, at least initially while you develop a method, sending out specimens to a lab that has the equipment to speciate the Cr. The instrumentation to do so is large, expensive, requires pricey consumables, and is a significant time commitment to maintain. It's really only sensible for a commercial lab or university to own and operate.
That being said... if your bath has a blue color when made up, I'd totally run a scan for the peak absorbance and then set up standards from your stock, in the same matrix in the tank of course, and check your results against those from a lab running GCMS. If you're looking for g/L actual Chromium content in the bath, rather than g/L (or %vv of the stock solution, as the conversion coating one I wrote spits out), you'll just have to have the lab actually analyze a sample of the stock solution, too- and then remember to correct for the actual Chromium content...
To do that you'd cross reference to the safety data sheet and determine what chromium compound contributes the Tri, and then determine the % of the actual Chromium IN THAT COMPOUND using the atomic weight of Chromium, how many atoms of it are in the formula, and then how that contributes to the overall formula weight.
If the safety data sheets weren't so intentionally vague, with a wide weight percent range that gives basic composition without revealing proprietary composition, or if you were getting all of your chromium from a pure industrial sack of Chromium sulphate, you could skip the lab testing entirely and build a method yourself.
Now, if you're talking about trying to find Tri CONTAMINATION levels in a hex bath, yeah... you're gonna want to send those samples out to a lab to be speciated. There's an EXTREMELY crude bath life test method given for Iridite 14-2 (a hexavalent conversion product) that involves carefully raising the pH of a bath sample to flocc the tri, and measuring the volume of the precipitate in the bottom of a graduated cylinder, because hex stays in solution while tri falls out, but when I say CRUDE... it's bad... in order to even apply something like this to your situation, and get REALLY gross approximation numbers, you'd need to build up months of data side by side with outside lab testing, and know if there is other significant metal contamination.
If you can find your peak absorbance for contaminant-level Tri, and are confident that nothing else in the bath would mask it (give a false-high reading due to absorbance at the same wavelength), go back to paragraph 2 and make sure your initial matrix you set up for calibration includes all components of the bath, at target concentrations, varying the amount of chromium from a trivalent source.
Titration for trivalent chromium, particularly in the presence of hex, is difficult and wildly inaccurate.
Good luck!

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
![]() Thank you Rachel! I am testing our chromium plating bath for trivalent contamination. I have arranged to send a sample out to a reference lab so I can determine what the bath value is now and so I can try match it with this procedure. Our plating line personnel want to be able to check the bath periodically for trivalent contamination but want an actual reportable value, hence the spectrophotometric method that I am pursuing. I have explained below what I have come up with as far as the prep of my calibration standards and bath sample. I would sure appreciate some input!
Thank you Rachel! I am testing our chromium plating bath for trivalent contamination. I have arranged to send a sample out to a reference lab so I can determine what the bath value is now and so I can try match it with this procedure. Our plating line personnel want to be able to check the bath periodically for trivalent contamination but want an actual reportable value, hence the spectrophotometric method that I am pursuing. I have explained below what I have come up with as far as the prep of my calibration standards and bath sample. I would sure appreciate some input!
I will be making up 5 calibration standards (0.5 to 7.5 g/L) from a stock solution (10 g/L) of Chromium (III) sulphate hydrate and adding approximately 12.5 g/L Chromic acid for matrix matching to each standard. I have seen several references to the addition of sulfuric acid as a buffer to prevent the reduction of Cr+6 to Cr+3. Is this also needed? I will use DI water as a blank & zero Spec at 600 nm, read the absorbance of each standard and make my curve.
Q. Like I said, I am not a chemist, I only play one at work!! I would appreciate any feedback I could get. Thank you!
- Peru, Illinois
February 24, 2021
In a titration for hex chromium, yes the sulfuric is necessary for the reason you stated. This is true when analyzing passivation baths, hex conversion baths, and dichromate seal for anodizing. And some doex/desmut baths.
In a spectrophotometry based method, match your matrix exactly, without the addition of anything you don't keep in the bath. You won't be doing anything to the specimens during analysis that would oxidize or reduce them. If they do change oxidation state between sampling and analysis, something has gone wrong, like contaminated glassware.
When you set up the calibration curve, though you're probably initially going to zero the machine on di water when it warms up, your "zero g/L" point when generating the equation will be the perfect mock brand-new tank sample. This corrects for a complex matrix effect.
I should add that there may be contaminants present in your bath that you aren't testing for, which is normal and unavoidable, that could potentially change how the chromium behaves vs how it behaves in your clean standards. If you send a specimen for outside testing and corroborate even within 10-15%, it's still more accurate than titration!
Join the club... my degree is in Fisheries Management and I only play a chemist at work... And have been keeping up the ruse for about 20 years so far. Don't tell anyone ;)

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
![]() Thanks again Rachel! Your input has been very helpful.
Thanks again Rachel! Your input has been very helpful.
Q. One more question...when you say "generating an equation" are you referring to generating the calibration curve graph or is there an actual mathematical equation that I am missing in my research? In past use of a spectrophotometric method, I have plotted my concentration vs absorbance curve and read off of the "curve" for the unknown's concentration result.
- Peru, Illinois
March 1, 2021
A. You'll derive your equation based on the curve ... that other post on this thread is applicable to all sorts of analyses. Hopefully it's self explanatory. Sorry a tree just took out my power lol "spring" in New England. Still gusting to 40 knots. Phone battery life is limited. Good luck!

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
A. Rebecca -- my response on the 23rd assumed that you were looking for an easy way to measure trivalent contamination in a hexavalent chrome plating bath (standard hard chrome or standard decorative chrome). Maybe I had misunderstood. If I did understand correctly, then my method does work and is accurate. I validated it against several other standard and instrumental methods. For the calibration standards, it doesn't really matter what you use for the trivalent- Chromium (III) sulphate is fine.

Jon Barrows, MSF, EHSSC
Kansas City
March 2, 2021
![]() You are correct Jon. I need to measure trivalent chromium as a contaminant in a hexavalent chrome plating bath. Thank you so much for your input regarding the layout of your method and the advice on the use of the chromium (III) sulphate. I have ran several methods using the spectrophotometer over my many years of lab work. However, they were always established methods with given calibration standards, curves, etc. Now that I have been tasked with coming up with one myself, I was lost on the specifics. I am very appreciative of your help and that of Rachel and this website.
You are correct Jon. I need to measure trivalent chromium as a contaminant in a hexavalent chrome plating bath. Thank you so much for your input regarding the layout of your method and the advice on the use of the chromium (III) sulphate. I have ran several methods using the spectrophotometer over my many years of lab work. However, they were always established methods with given calibration standards, curves, etc. Now that I have been tasked with coming up with one myself, I was lost on the specifics. I am very appreciative of your help and that of Rachel and this website.
- Peru, Illinois
March 3, 2021
A. Jon, you've got a good solid method. Rebecca, that how-to-make-a-curve chart is handy and I hope you find it useful for many applications beyond this. As a humorous aside, my annual performance review just came back with a note to stop over-explaining things and avoid writing status updates and SOPs that read like the unabridged version of War and Peace, so... I just have to laugh, and step down.
Apologies for the extra explanations. I hope it had, at least, been an interesting read :)

Rachel Mackintosh
Lab Rat / WWTF - Greenfield MA
Long-winded lab rat (Seriously. I'll own it.)
![]() Own it proudly, Rachel. One of the few useful things I learned from management and training courses is that the biggest problem in most businesses is inadequate communication :-)
Own it proudly, Rachel. One of the few useful things I learned from management and training courses is that the biggest problem in most businesses is inadequate communication :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Jon & Rachel, thank you both so much for your help with this project of mine. You have no idea how much I appreciate your knowledge! I received the results of the trivalent chromium analysis of our plating solution that I sent out to an outside lab. The result came back at 2.61% which I hopefully converted correctly to 26.1g/L and 3.485 oz/gal. I am having trouble finding literature that will tell me if this result is out of a normal range and considered a contaminant. Could you help? I have found ranges in some literature that didn't give me the units that it was reported in, so that didn't help. Thank you!
Rebecca Batty [returning]- Peru, Illinois
April 21, 2021
by Robert K. Guffie

on eBay (rarely) or Amazon (pricey)
or AbeBooks (rarely)
(affil link)
I am skeptical that your analysis value is really that high. Trivalent contamination should be as low as possible, but certainly less than 4 g/L or so. If it is as high as you say, then you would be experiencing major issues. That level of contamination would be caused by some sort of incorrect operational practice. It could be insufficient anodes, passive anodes, excessive reverse etching or a few other things or combinations of things. It is a little too much of a dissertation to get into through this forum. I suggest that you take NASF's Chromium Plating course which is available online. If you can find an old copy of Guffie's "Handbook of Hard Chromium Plating", that would also help. Either of those would give you the tools you need to identify the cause as well as correct the bath so that you can start using it again. Good luck.

Jon Barrows, MSF, EHSSC
Kansas City
Q. Thanks Jon! I will look into acquiring that book. Could I have miscalculated the grams/liter? The result coming from the testing lab was in percent. I just used an online calculator to come up with the 26.1 g/L. Am I missing a step or is this correct? Do I need to factor in density or anything else? I will be using g/L to make up the standards for my trivalent chromium method so I was really hoping to find at which point our bath is "contaminated" in g/L. Sorry to bother you with all of this. I really appreciate your help!
Rebecca Batty [returning]- Peru, Illinois
April 22, 2021
Q. Hello Jon,
I tried your spectrophotometer method for the determination of trivalent chromium. This method is very user friendly and would be perfect for what I need. However, I am having trouble matching the results that were obtained from the lab that did our outside trivalent chrome analysis on our chrome plating bath. I have briefly outlined my procedure and was wondering if you could tell me if I am missing something.
Prepared a 10 g/L Stock solution of Cr2(SO4)3 and made 5 standards from it (0.5/1.0/2.5/5.0/7.5/10 g/L).
Prepared a 250 g/L solution of CrO3 to use for matrix matching and added 5 mls to each of my standards and blank.
Analyzed absorbance at 600nm for the blank and each standard using the spectrophotometer.
Filtered a sample of our chromium bath plating solution through a #40 filter paper and analyzed for absorbance.
Had to make a 20:1 dilution in order to obtain an absorbance reading within those of my standards.
Plotted absorbance vs. concentration using the old fashion graph paper and obtained a pretty nice looking linear graph. However, when I read my absorbance for my sample and multiply by the dilution, I am nowhere near to the value obtained from the outside lab (26.1 g/L). I know that this value is extremely high but it's the only one that I have to work with. I am actually reporting approximately 120 g/L at my 20:1 dilution.
Could you look over this procedure and let me know if I am doing or using anything different from your trivalent chromium procedure that you referenced in this blog? I would really appreciate it. Thank you.
- Peru, Illinois
May 11, 2021
⇦ Tip: Readers want to learn from your situation;
so some readers skip abstract questions.
Q. For chrome baths, do you have any titrimetric or spectrophotometric method to determine Fe and Cu?
Student - Romania
September 29, 2021
Q, A, or Comment on THIS thread -or- Start a NEW Thread
 on eBay
on eBay