
-----
Testing Silver at Home by the consumer
Simplest tests include magnet, Archimedes test, heat conductance, bell-like ringing, mustard stain test, and vinegar ⇦in bulk on eBay or Amazon [affil link] test -- but several different tests must be done. Acid test is best!
< Prev. page (You're on the last page of this topic)
Q. How do you tell the difference between solid silver and silver solder?
Kayla Ferguson- Spooner, Wisconsin, USA
April 14, 2013
A. Hi Kayla. Solder is for joining things, not making things. It might be used to connect a cup to a base, for example, or a handle to a teapot -- but a cup or base or handle or teapot would not be made out of solder. At least that's my understanding.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi there, I have an antique concho belt bought at an estate sale. Each medallion is stamped on the back "madeinspain" with a capital "E" in a circle also stamped on the back of the medallion. It has tarnished, black does come off when wiped, could it be silver? Thank you for your response.
Stephanie Benbenek- Greer, South Carolina
May 6, 2013
A. Hi Stephanie. It could be, but I doubt it. I'd guess tin or pewter, assuming it's non-magnetic. Black tarnish is certainly not exclusive to silver, and silver tarnish doesn't usually wipe off easily. I think the overall thrust of the thread is that testing will beat guessing, and multiple types of test beat a single test :-)
Good luck and Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 9, 2013
Q. I have like 4 canisters that stack and sit in a holder with Chinese or Japanese wording, hallmark is a seal with ball on nose. Magnet does not stick to them
Penn Tinklenberg- Milaca, Minnesota, US
October 11, 2013
A. Hi Penn. There are hallmarking books in the library if you wish to try to figure out who made this set. But the fact that they are not magnetic only says they're not steel. They could be stainless, tin, zinc, copper, brass, pewter, silver-free "nickel silver", silver ... almost anything. You need to run additional tests if you wish to know what metal these are made of. good luck!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
October 2013
Q. I have been given two silver coffee/tea sets with sugar and creamers. Both are very old. Great grandparents on both side. I am in my 60s. One says made by Rogers and has a name called Princess and has a long number carved in it. Not the 92....whatever.
The other has a name like Belkin or something like it and nothing else.

I need to figure out if it is Silver. Where can I take them?
Paula Houghm- Mpls, Minnesota, USA
October 23, 2013
A. Hi Paula. The simplest test is to see if they say "Sterling". If they do, they're silver. If they don't, the chance of them being silver is fairly slim, and Rogers made far more silverplate than sterling. Unfortunately silver plated holloware, with few exceptions, is pretty much valueless at present -- save it for your great granchildren.
I see "We buy gold" signs on many street corners these days; I think any of those places can identify silver as well. Also, an experienced antiques dealer might have some knowledge of these. Even sterling isn't worth a lot; browse eBay [adv: item on
eBay] and try to find some similar items. Better to try to enjoy them than to try to sell them :-)
Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
October 23, 2013
Q. I found a tarnished bread tray at a thrift store. The hallmark is for Webster and Sons. It is a star within a web. The model number is 109. The tray is tarnished and appears to be silver or plated. There is no sterling or plated markings on it. The tray is very soft. I can put a bulge in it by pushing with my thumb. It was warped when purchased, but I easily twisted it back into shape. Scratches do not reveal a different color underneath. This tray really has my curiosity peaked. If this is a alloy under the plating, any ideas what it would be?
Andy Janes- Moncton, NB, Canada
January 17, 2014
A. Hi Andy. If you search eBay for Webster and Sons [adv: item on eBay], you'll see generally similar items with the same star within a web. It is probably silver plated nickel silver (nickel silver contains no silver). Hopefully you like it because it's really not valuable or saleable. Good luck with it.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
January 20, 2014
Q. I am trying to figure out if a rock is real silver.
Trinity Amanda shaw- Moosup Connecticut United States
March 26, 2014
Hi cousin Trinity. You probably feel that all of the previous 3 dozen postings are inapplicable to your rock, and you might be right. Still, it's best to explain why in your question :-(
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi I have a pair of plain corinthian column candlesticks about 7 inches tall. They appear to be silver. There is no wear to a base metal. The markings are obscure and I can't trace them anywhere. There is what appears to be the initials AB in old scrolled victorian script and the second mark is An A over an O in a shield.These are stamped into the side of the base area. I have done the bell ringing and the vinegar test with good results. Can you enlighten me further?
John Dodd- ipswich suffolk great britain
July 16, 2014
A. Hi John. If you can send a pic of your Hallmarks there is a slim chance someone will identify it. From a verbal description, unfortunately no chance (from my experience posting hundreds of these messages for 20 years now). Candlesticks are usually weighted, so even if they do turn out to be sterling, you probably can't get a scrap evaluation until they're broken open and ruined, so try to just enjoy them :-(
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
July 2014
July 16, 2014
Q. Thank you for your reply. These are not weighted. They are cast and hollow but quite heavy. Just done the ice cube test and this melted extremely fast leaving the sticks ice cold within seconds. Someone has scraped deep into the base of one revealing that they are one solid white metal.




Actual dimensions are 15 cms by 8 cms so not English. Could be some obscure Continental mark. They look early Georgian to me, possibly around 1760.They have removable drip inserts at the top. Lady told me they came back from Germany during WW2.
John ;)
- ipswich suffolk great britain
Q. Found a silver ring and wondering if it's worth keeping. I tried the magnet and also the pencil method but the ring is too thin and too small to tell. This silver ring has a letter NV on the other side and a letter S on the other side, both on the inside of the ring. I have no doubt that the diamond on it is not real but still want to make sure if the silver ring is real.
Lala Fletcher- Anchorage, Alaska,United States
December 31, 2014
A. Hi Lala. This thread is about how to test silver, rather than about how to identify silver from its markings, so we haven't repeated this before on this page: there is no hope at all of a reader identifying your ring from a verbal description of the markings. We have half a dozen pages about hallmarks, and with hundreds of such postings and a million readers, nobody has ever identified a single one from such lettering in 20 years now -- sorry, it's not going to happen :-(
That means, unfortunately, that you're not going to know whether it's silver or not unless you do some more tests. Apologies for the bad news, and good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
December 2014
Q. Mystery Set of Very Old Candle Sicks - Silver? Maker? Era? Marking?
Have come into possession of a pair of heavily patina candle holders. I have looked in vain to find any marking. I have only done the ice test and melts very fast. I do not want to try the chemical test due to ruining the patina.



If anyone may have any information as to a possible maker, age, metal content or anything you may know, please respond. It would be very helpful.
Keith WolkAppraiser - altamonte springs Florida. USA
January 6, 2015
Q. Hello Sir,
I have tested a piece a silver with help of acid, by rubbing on a stone.
It turned to cream colour /milk colour, but when I gave it to an assayer, he confirmed purity of silver as 68%
Whether I am correct?
Tell me a proper method to identity it?
- India
November 14, 2016
February 24, 2017
A. So I am done quite a bit of testing and have concluded that the bleach test is the best for determining if an item is indeed silver.
For control, I used a 1959 Quarter (known 90% silver 10% copper). As test subjects I used 3 spoons that I thought were silver. Later I tested a necklace I bought at Walmart (labeled silver) and a cross stamped (sterling).


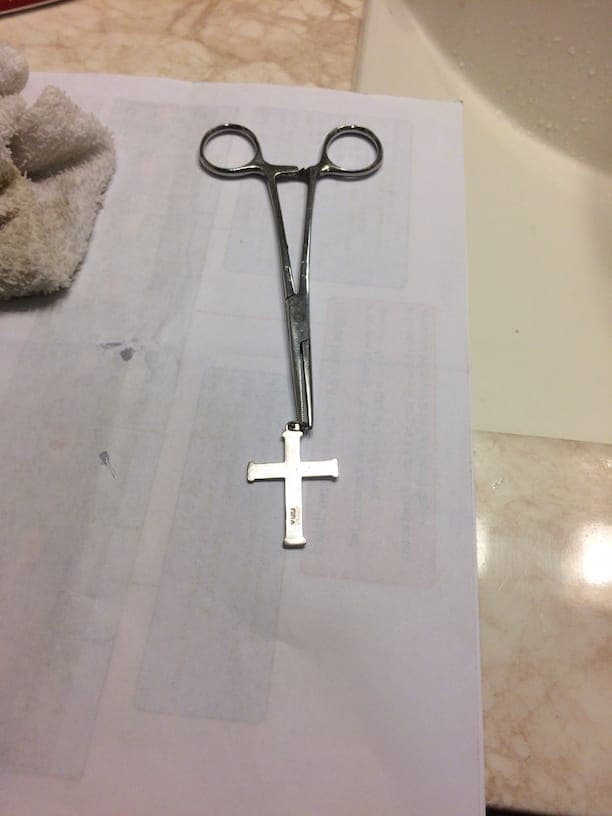
With the spoons, using a stainless steel spoon for control, I tried testing: magnetism, heat conductivity, electrical conductivity, weight differential and bleach. Bleach was by far the best definitive test.
All subjects were non-magnetic. Putting each spoon in a cup of ice water and measuring the temp 6.5" from the tip of the spoon was inconclusive. Measuring electrical resistance of ohms over 4cm was inconclusive. Measuring the weight of 2 quarters, 1 nickel and 3 dimes (known silver) against modern equivalence was too close to call.
However, the bleach test is very conclusive. Attached is a picture of the 3 spoons ("Royal Plate Co", "Annette Silvore", "Brazil Silver") in question, along with the 1959 Quarter, after each were partially submerged in bleach for 5 minutes. (to note I had a handful of random modern coins in the bottom of the bleach vessel for stability, in case there is concern of that), (I also believe 2 minutes is probably sufficient and most likely less damaging to the test object).

You can see that 2 of the spoons were hardly effected by bleach but the 1959 Quarter and the "Royal Plate" spoon turned "Black".
This did put me in a bit of a panic but I was able to clean off the "black" with silver cleaner. In fact, if you look at the "Royal Plate" spoon after cleaning, the "bleached" section is "cleaner" than the rest of the spoon.

I think I will re-dip the entire spoon after I get some new cleaner (as I am almost out).
After this first test was complete I tested a necklace I bought from Walmart (labeled silver, sterling I think), and cross stamped "Ster" for sterling silver I believe.
I think silver jewelry nowadays is either coated with something, or mixed with Germanium, to help prevent tarnishing.
Testing the necklace and cross you can see they both turned "black" but not as definitively as the spoon or quarter. Particularly with the cross, it looks as though there is perhaps a layer of something trying to protect the silver. But there is definite "blacking" to show the object is made from silver.

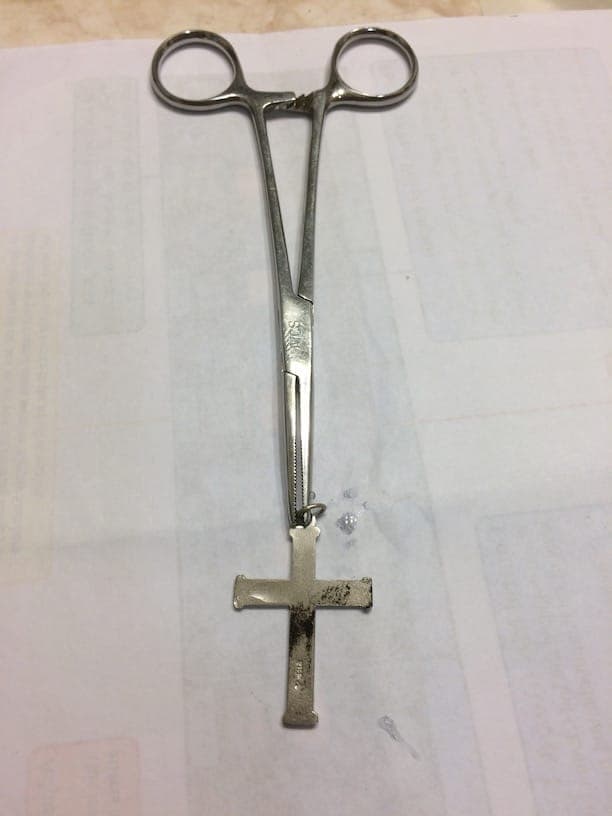
FYI, in the pics of the necklace you can see the "vessel" I used for bleach. There is also a picture of the necklace soaking in vinegar in hopes to remove the "blacking" but that did not work. For me, only silver polish removed the "black" and it takes "A LOT" of effort.

In conclusion, I found the "bleach" test to be very definitive but I would be conservative on the length of exposure to limit the "clean up" time from the test.
Blessings
Greg
- Inver Grove Heights
Why does silver tea set remain slightly 'goldish/yellowish' despite endless polishing?
Q. Hi I have a tea set with no markings on it; I have done the ice cube test, match test, bleach test, you name it I have done it and they all pass. However, even after cleaning and cleaning they still have a gold-ish tinge to them. Very faint. Any ideas as to what this mystery metal could be or is it possibly silver?
Nene CineseHouse wife - Vaughan, Ontario, canada
January 18, 2019
Q. White metal alloy passed all home tests for silver: magnetic, ice cube, ring, and silver solution and yellow brown color. And 18 kt acid don't turn milky white, but line stays and 22 kt takes it out. And I believe it is lapidary scrap containing 60 percent silver and possibly gold and aluminum because specific gravity is off from silver, and aluminum and gold getting too heavy for aluminum and too light for Pure silver and testing positive on 18 karat acid without turning milky white.
I am believing that it's got aluminum and gold in lower KT and the 18 KT acid not turning milky white makes me think it could be 6 KT inquarted for later acid refining by the lapidary scraps. I acquired from a friend who used to be a diamond cutter and jewelry maker and I know it is not plated and has a gold-ish white color and can see at the end of bar casing the metal is solid and not plated and gold.
Hobbyist - Tulare California USA
September 20, 2019
----
Some related finishing.com threads which might interest our readers:
Topic 42765 Confused about "acid test" for silver and other precious metals
Topic 34808 Testing purity of silver
Q, A, or Comment on THIS thread -or- Start a NEW Thread