-----
Chrome plating mist /fume control: PFOS, fume suppressants, surface tension reducers
Chrome plating is a very 'inefficient' process usually done from a strong solution of chromic acid. It generates lots of hydrogen gas, bubbling up in champagne fashion carrying tiny droplets of chromic acid with it -- and most governments consider chromic acid carcinogenic.
For many years the answer was to use a 'fume suppressant', an oil lying on the surface of the chrome tank, limiting the escape of those droplets. This in turn became an issue because the material best able to survive in chromic acid is PFOS, a 'forever chemical' believed likely to cause androgyny in fish and other problems.
RFQ: Hi does anyone here happen to still have a small amount of Fumetrol 140 in their possession? I'm looking for a very small sample of this stuff (originally marketed by Atotech but out of production for some time now). I only need a few milliliters at most, and I can pay for it and any shipping costs as well.
Actually even an empty bottle would be enough. I only need enough to use as an analytical reference material, so the residue inside an empty bottle would give me sufficient amount.
Thanks in advance!
- Durham, North Carolina
March 5, 2025
privately respond to this RFQ
Ed. note: As always, gentle readers: technical replies in public and commercial replies in private please (huh? why?)
A. Chrome fume suppressant,
If you have MacDermid chemistry, ask to MacDermid salesperson. They have chrome fume suppressant; need to add small amount. This chemical control fume and surface tension down.

Popatbhai B. Patel
electroplating consultant - Roseville, Michigan
A. Atotech disposed of all the Fumetrol 140 in the lab.
I used to work there and all Pfas/Pfos material was disposed of.
It's been a few years since they last sold this material. You could try to contact the supplier? Bayer, to see if they have any of this material available.
- ROCK HILL, South Carolina
![]() Hi George, thank you very much for the reply. I suspected that Atotech likely disposed of all the PFOS-containing formulations years ago. I have reached out to Bayer to ask if they have any of the original formulation remaining. Here's hoping.
Hi George, thank you very much for the reply. I suspected that Atotech likely disposed of all the PFOS-containing formulations years ago. I have reached out to Bayer to ask if they have any of the original formulation remaining. Here's hoping.
Funny coincidence, Rock Hill SC is actually my hometown. I had no idea Atotech was based there until this year.
All the best,
Lee
- Durham, North Carolina
April 6, 2025
⇩ Related postings, oldest first ⇩
Q. We have a small hard chrome plating operation and have been planing to install an air scrubber to comply with the chrome emissions standards. Recently we received information that suggests that hard chrome platers can achieve the emissions limits by use of fume suppressants alone. We called the company that supplies the suppressants and they could not tell us if anyone is attempting this. Does anyone know if other platers have tried this approach and were they successful with it?
Jim Klein- Grants Pass, Oregon
1996
A. I have previously discussed this and other issues with Mr. Phil Mulrine of EPA's RTP. At that time a fume suppressant that met 30 dynes /cm3 was proposed to be RACT (reasonable available control technology). This information is not current so you should check for updates. You might find more current information from :
members.aol.com/ryoung7547/elenvnav.html ⇩
Also, if you get flagged for "steel pickling" MACT due to electroplating you can suggest that this was not intent of CAA amendments. This has been successfully achieved with EPA previously.
Ed. update: The above link is broken. Thankfully, the Internet Archive preserved a copy here ![]()
Please consider a donation to The Internet Archive.
Q. I am in the copper engraving field of printing and on long runs of printing we chrome face our copper dies to make them last longer. I have been reading on some of the dangers with chroming and we are looking into maybe sending our dies out to be chromed.
I have spoken with a few bumper shops here in town and have been told that they can't do what I need. I have however noticed that a few of them have no exhaust scrubber on their systems and when I asked them why they didn't have one I was told that they used an additive that reduced the fumes. Is there such a thing? Where would I get it? Thank you.
- Smyrna Georgia USA
2000
A. What they are using and referring to is a fume suppressant and/or a surface tension reducer. The fume suppressant forms an oily foam blanket on the surface and is usually a fluorocarbon. Reducing the surface tension of the chrome plating bath offers the evolving hydrogen bubbles less capacity to form bubbles to carry plating solution out.
You can talk to DuPont or to any local distributor of plating supplies.
Please don't read this response as an affirmation that the use of a fume suppressant and/or surface tension reducer without an exhaust scrubber is always adequate because:
1). First you have to determine if you are a 'decorative' chrome shop or a 'hard chrome' shop; they may use different suppressants.
2). You not only need to use the suppressant, but follow the rules for testing and recording your results.
3). Rules are in a state of flux, and local codes may override federal rules anyway.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
for Shops, Specifiers, & Engineers

by Weiner & Walmsley (1980)
avail from eBay, AbeBooks, or Amazon

by Robert K. Guffie (1986)
avail from AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon
"Hard chromium plating: A Handbook of Modern Practice"
by John David Greenwood (1971)
avail from eBay

very rarely avail from Amazon
but copies are available in a few libraries)
"A Chromium Plating Bath With The Fluoride Ion"
by Alfred Perlenfein (2013)
avail from eBay, AbeBooks
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
Q. Dear all,
I work for a plating shop and currently we are facing problems with the fume suppressant. In detail we have used fume suppressants in a hard chrome bath from two different suppliers, one of which is a major international chemicals company. Both liquids do not perform as good as expected. For example, after no more than 14 hours of operation everything near the bath is full of this dark red/brown chrome layer. If you leave a white page of paper in the afternoon on a table 2 m from the bath, next day it has become brown.
Should you have any suggestions, please reply.
Thank you in advance.
- Athens, Attica, Greece
2004
A. Personally, I don't feel that fume suppressant is always an adequate substitute for an exhaust ventilation system, but your main problem may be that need to also control the surface tension. But try dropping the solution level in the chrome tank to whatever extent is possible as well -- it can make a very big difference. I once visited a plant that had been built with very deep tanks for vertically chrome plating navel gun barrels. When they no longer processed parts that long, they decided to drop the solution level to at least 36 inches below the tank rim. Without fume suppressants or a ventilation system, they had no detectable chrome emissions! Apparently, in the absence of drafts, the chrome mists fell back into the tank before rising this 36 inches or so. That much freeboard is impractical for most shops, but I think it indicates that every inch of freeboard makes a big difference in emissions.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Hi Christos,
What Ted said about ventilation hit the nail on the head ... and yes, the lip of the tank should be min. 6" (sorry, I forgot ... 15 cm!) from the liquid level.
I have seen very poorly designed fume hoods inefficiently exhausting a chrome tank but with the addition of some surfactants, they became 'efficient' !
If this Atotech material works that efficiently, doesn't cause any side effects, is reasonably priced, then it will prove a huge benefit to platers.
But as a typical Doubting Thomas, me thinks it ain't so.

Freeman Newton [dec.]
R.I.P. old friend (It is our sad duty to
advise that Freeman passed away 4/21/12)
A. Fume suppressants (foam blankets) are not very effective and are short lived however, surface tension reducers are very effective in eliminating chrome mist and spray (as has been proven in several tests by U.S. EPA et.al.) If you are using one of the modern fume suppressants/surface tension reducers check to see if your surface tension is below 45 dynes (I prefer 35). This may be done with some accuracy using a relatively inexpensive piece of glassware called a stalagmometer.
Gene Packmanprocess supplier - Great Neck, New York
A. We have used a fume suppressant for ten years, and it works very well. There are no fumes you can see or smell, and there are no chrome stains on or near the tanks. The one we use is available from Atotech USA.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
Thanks, Jeffrey. To be clear, you do hard chrome plating with no local exhaust on the tank, and do not have an unusually low solution level in the tank, and need nothing but fume suppressant for control? I worked for M&T Chemicals and Harshaw Chemical Co., the two components of Atotech decades ago, and am happy but a bit surprised that the development efforts were that successful! If you are at liberty to say, do you use conventional sulfuric catalyzed, fluoride, or high efficiency etch free chrome?

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Ted: Our chrome bath is sulfuric/boric acid catalyzed, no fluoride. approx. 32 oz/gal, solution level 4" from top. No ventilation of any kind. We have done 8-hour employee air monitoring with no Cr+6 detected. Also installed a temporary exhaust system, no scrubber, just a blower and ductwork, and passed the Cr emission test by a factor of 10. You can lay a white paper on the anode/cathode bars, and no yellow stain @ 2000 amps. The product is Fumetrol 140. Worth it's weight in gold, which is almost what it costs.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
![]() Thanks Jeff!
Thanks Jeff!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. I have had the same results as Jeffrey in our shop.
The problem is companies want to keep on selling scrubbers, regulators want more regulations, and a plating society was willing to go along for the ride. Scrubbers were embraced, surface tension reducing agents were shunned. It was an excellent job as people to this day will denounce surface tension reducing agents.
Todd Osmolski- Charlotte, North Carolina, USA
![]() Dear friends,
Dear friends,
Thank you all for responding. In fact, one of the two products I mentioned is Fumetrol 140!
We do use an exhaust system and the space above the solution level is about 30 to 40 cm.
Well, the problem probably seems to be that the filters of the exhaust system, which is situated at the basement of the shop (under the bath), have to be changed more often because they were blocked.
Anyway, thanks again, pretty interesting discussion.
- Athens, Greece
Q. Hello,
Is there a good combination of F/S, and surface reducing agents. I have 10" of freeboard, use Fumetrol 208 and have an exhaust system. After a night load I get the "Red Mist" on all my bars. Tried the balls, sank. Thanks
hard chroming - North Bay, Ontario, Canada
2004
A. Jamie,
If memory serves me correct Fumetrol 208 is a cost engineered product that contains less of the surface tension reducer and more surfactant. Switch to a product which is mostly surface tension reducer. These are relatively quite expensive ($85-160 U.S. depending upon vendor/manufacturer) but are based on fluorocarbon technology.
process supplier - Great Neck, New York
Update on PFOS, etc.
Q. Hello
I work as an environmental engineer for a customer, operating a traditional hard chrome plating line. At this moment, he uses a mist suppressant based on PFOS (a fluorinated compound). Products based on PFOS will be either banned or heavily regulated in Europe.
We are comparing two options:
- stop using mist suppressants and build an expensive exhaust system for evacuation of the chromic acid mist
- continue to use PFOS, but remove the PFOS from rinsing water before (or right after) the sulphite treatment (conversion of Cr(VI) to harmless Cr(III)).
Does anyone have experience with removal techniques for PFOS. For example: does active carbon adsorption work ? (We found out that 3M uses active carbon to remove PFOS from polluted ground water, but does it work on highly acid rinsing waters ?) Suggestions and ideas are most welcome.
- Gent, BELGIUM
2006
A. If it is possible, what I would do is plan for a large amount of freeboard plus the exhaust system. Chromium mist droplets are heavy, and with extravagant freeboard much of the chrome will fall back into the tank and not need to be exhausted.
We're going through the same issue in the USA. With only a little exaggeration, EPA no sooner claimed that chrome MACT standards were achievable and affordable through the use of fume suppressants than it began banning them. But PFOS and related chemicals are getting scary as we see them directly causing androgyny in fish :-(

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Hi Bert,
What Ted said about banning suppressants is news to me ... but his remarks about 'freeboard' are interesting ... I used to think that a 6" (sorry! 15 cm) freeboard was OK.
On the subject of, you say, an expensive exhaust system ...
why expensive?
Welded mild steel ducting and hoods. Anyhow, go into the fin.com library for more data on that.
POLLUTION ... Back in the early 80's, the allowable specs for chrome in B.C. were ll grains/l000 cfm of chrome sulfuric acid. Using a well designed (and made) ordinary 2 stage PVC mist eliminator, horizontal design with sine curved blades, tests indicated that these were 30 times LOWER than the then acceptable limits.This is a l2 micron eliminator...but in your terminology that is 0.7 mg/M3
My 1974 design followed that route but was a good 3 micron inertial scrubber.

Freeman Newton [dec.]
R.I.P. old friend (It is our sad duty to
advise that Freeman passed away 4/21/12)
Q. Ted, Freeman,
Thanks for the input.
Increasing the freeboard is a nice idea; in this particular case, the freeboard can be increased by ~25 cm (some 10") just by lowering the level of the tank.
Also thanks for confirming that a well designed mist eliminator will do the job. (This still leaves us with the cost of a new exhaust system).
Does anyone has experience with e.g. ACTIVE CARBON ADSORPTION of the chlorinated mist suppressors ?
Best regards
Bert
- Gent, Belgium
A. Activated carbon might work. But, frequently, charged ions like the salts of anionic surfactants have a higher affinity for anionic ion exchange resins. These compounds are going to be even more acidic than non-fluorinated surfactants of this type and will only be present as the free acid at very low pH values.

Dave Wichern
Consultant - The Bronx, New York
Q. Dear Sir
What is the emission limit for open tanks during hardchrome plating?
Note that We are not using any fume suppressant in our tanks.
please help!
Best Regards
Assistant Manager - Karachi, Pakistan
2007
A. Hi Mohsin,
The U.S. specs for chromic emissions are very tough, i.e., 0.05 mg/cu M....maybe too tough.
The specs back in l978 in B.C. were ll grains of sulfuric/chrome per 1,000 CFM (or was it per single CFM, I forget).
Using an ordinary but very well built CT-120/2 blade type eliminator, we achieved 0,34 grains .... that's equivalent to 30 times lower than those specifications BUT translated into metric, it's only O.7OO mg/cu.M Yes, I have that in writing.
You definitely need some exhaust.
Does this help?

Freeman Newton [dec.]
R.I.P. old friend (It is our sad duty to
advise that Freeman passed away 4/21/12)
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. What is the status of chrome plating fume suppressants / mist suppressants that do not contain PFOS (perfluorooctane sulfonate) or fluorinated surfactants?
Are they available?
what is their performance like?
What surfactants are they based on?
what are their strengths and weaknesses.
Is anyone using one and willing to share their experience with it.
A MN plater was identified as the primary source of PFOS going through a medium sized POTW, causing the POTW effluent to exceed a "health based value" set by the state health department.
As a result there is some interest on the part of local platers to begin looking at alternatives if they exist.
resource to the MN metal finishing industry - Minneapolis, Minnesota, USA
2007
A. Based on the usage of the fluorinated products, I can't figure out how so much got to the POTW that the POTW was high!
Gene Packmanprocess supplier - Great Neck, New York
Q. We are talking very small amounts here.
The health department standard is 75 ppt for PFOS.
The POTW effluent was 12 times higher than that.
There was no attenuation in the POTW, and the plating shop effluent was 58 time higher than the POTW effluent.
All this is based on a relatively few samples.
MnTAP / University of Minnesota - Minneapolis, Minnesota USA
A. Since this product is getting into the effluent via drag out from the plating solution, the most effective way to limit this is to reduce the drag out in the plating facility by implementing waste reduction methodology.
Gene Packmanprocess supplier - Great Neck, New York
Q. We know wetting agent in Bright Nickel is SLES (sodium lauryl ester sulphate),
Can we use it in bath of Chrome plating as wetting agent?
Thank you, Good luck.
plating shop employee - ARAK, IRAN
November 6, 2010
A. Nope, chrome solutions would destroy that in no time. If you use surface tension reducers in your chrome bath, you need the ones that are specifically designed for chrome plating. The chemical suppliers that offer these additives have spent big bucks on R&D to solve this particular issue for you, so the additives are expensive. Until recently most suppliers offered PFOS or similar compounds, but those chemicals are becoming banned, so they are now offering brand new proprietary additives.

Jon Barrows, MSF, EHSSC
Kansas City
A. We were just asked by the Air Quality folks to lower the emissions of our chromic acid anodizing tank. We are looking into some of the Fumetrol products. I see that they have a new product that is PFOS-free.
Kevin ClarkAerospace - Monrovia, California, USA
Q. What is the best way to stop evaporation from my chrome plating tanks? If it is to be purchased, where do I get the product?
Dave Kiminkiplating shop - Dallas, Texas
April 7, 2011
A. Hi, Dave.
Evaporation, per se, from a chrome plating tank is almost always considered good rather than bad as it allows the return of some chrome-contaminated dragout.
The normal problem is chrome fumes or mists evolving from the tank which should not be confused with evaporation. These can be addressed with a lowered solution level in the tank, wetting agents, and fume suppressants. Some people use "chroffles" (polypropylene ping pong balls), but I am not convinced they are worth the effort.
You might also see www.pfonline.com/articles/reducing-hexavalent-chromium-emissions ![]() about a special membrane cover which purportedly allows hydrogen and oxygen to escape from chrome plating tanks while containing the chrome fumes. Good luck.
about a special membrane cover which purportedly allows hydrogen and oxygen to escape from chrome plating tanks while containing the chrome fumes. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
 Ed. update March 2025 : Unfortunately, yet another formerly surfable website has bitten the dust, as the original promise of a freely readable internet fades to black, and site after site can now only be viewed after registering with your personal information.
Ed. update March 2025 : Unfortunately, yet another formerly surfable website has bitten the dust, as the original promise of a freely readable internet fades to black, and site after site can now only be viewed after registering with your personal information.
Finishing.com has always been and will always be no registration, anonymously readable in its entirety.
Q. According to ASTM D1331, you must calculate the correcting factor F, when using a tensiometer. How do I obtain the value of "d", which is defined as the "density of air saturated with vapor of the liquid"?
The ASTM spec says the value of "d" can be obtained from published data, but I have not found anyone who knows this data or how to get it?
This is in regards to decorative chrome solutions.
Thank you, Jeremy.
- Portland, Oregon, USA
December 28, 2011
A. You might have better luck using a stalagmometer. There are several companies that sell this instrument and I'm sure they could provide you with a procedure that would work for you. Tensiometers are a big pain to use because there are many sources for error. And as for a literature source, I would check the International Critical Tables for a value, it might be in there.
Aimee Longacre- Savannah, Georgia, USA
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. Dear sir,
Which chemical is used to control the hydrogen gas in hard chrome plating? Please suggest to me. I use Mistolin but it is not working truly and its price is very high. Please tell me about other chemicals which are used to control the hydrogen gas.
hardchrome electroplater - vadodara, gujrat, India.
March 4, 2013
Q. I support the Plating Shops at a Naval aircraft overhaul facility in Jacksonville, Florida. While researching ways to reduce chromium air emissions (due to misting), I found this page. The first response is Ted Mooney's:
". . . try dropping the solution level in the chrome tank to whatever extent is possible as well -- it can make a very big difference. I once visited a plant that had been built with very deep tanks for vertically chrome plating naval gun barrels. When they no longer processed parts that long, they decided to drop the solution level to at least 36 inches. Without fume suppressants or a ventilation system, they had no detectable chrome emissions! Apparently, in the absence of drafts, the chrome mists fell back into the tank before rising ha 36 inches or so. This much freeboard is impractical for most shops, but I think it indicates that every inch of freeboard makes a big difference in emissions."
Can anyone provide more information about the plant mentioned above that implemented this change? Could you give any POCs there that may be able to answer questions about what they did and how their EPA- and Permit-types reacted?
Our hard chrome workload has changed to primarily small parts, possibly allowing us to get away with lowering the solution levels in our 8-foot deep tanks to give the ~36-inch freeboard. And possibly eliminate/reduce the costs and problems with our chrome scrubber system.
Can anyone offer advice or suggestions about any of this? Has anyone other than the above plant implemented such a change? If so, what were your results?
Thanks,
Plating Shop Process Engineer - Jacksonville, Florida, United States
April 4, 2013
A. Hi Bob. It was a very long time ago, mid 1970s, and I was not involved in the chrome fume control. I was visiting on a different matter but inquired when I noted the very low solution level. One of the plant's engineers explained the situation and demonstrated it by holding a white handkerchief over the tank. But I have no data or history on it. I sent you the name of the shop in private.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. We use Fumetrol Suppressant from Atotech but the fumes are not getting reduced many times.
Any other options available to reduce the fumes?
Please let me know?
Our current levels are between 2800 to 3200 Amps.
Thanks,
Plating shop manager - India
November 11, 2016
A. Good day Ram.
Have you checked for surface tension with a stalagmometer?
Regards,
Aerotek Mfg. Ltd. - Whitby, Ontario, Canada
A. The most common and reliable alternative to any fume or mist suppressant is a well designed Local Exhaust Ventilation system integrated with smart tank covers that completely capture and control the mist/fumes, reduce heat loss, reduce evaporation, and most importantly reduce your electrical bill! With the banning of PFAS>PFOS>PFOA based fume suppressants, many forward thinking operations already rely on these systems, which can completely pay for their initial investment over a period of only a few years through proven energy savings. Over a 25 year life-cycle of the equipment, smart tank covers will have save hundreds of thousands of dollars in medium to large process line operational costs.
Kyle Hankinson- Forest City, North Carolina, USA
April 26, 2018
Q. Dear expert, I have a Nickel Hard Chrome Plating shop. During process for collection of Chromium fumes on Hard Chrome tanks provided suction duct & connect to F.T.D. (Fume Treatment Device with Water spray). I would like to know:
1) How to reuse (or what is process of reuse) waste chrome which is collected in FTD unit?
2) How to minimize/control waste chrome?
a) Density of waste chrome is near about 55 - 60 Deg Baumé.
b) Impurity level is high.
c) Chrome solution is like slurry base.
- Aurangabad, Maharashtra State, India.
April 6, 2019
A. Hi Rajkamal. We attached your inquiry to a fairly long thread on the topic because there aren't easy answers, there are only balancing acts :-)
1. Re-use might be possible by replacing evaporation losses in the chrome tank with recirc water from the fume scrubber ... but more efficient fume scrubbing results from including sodium metabisulfite in the recirculating water to convert the hex chrome to tri chrome, and that would render it non-reuseable.
2. More freeboard, tank covers, and use of fume suppressants and surface tension reducers minimizes the waste, but fume suppressant can be an environmental problem itself. If 'impurity level is high' what are the impurities? Sorry, I don't know what you mean by "like slurry base"."
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Typically, metallic impurities in the washdown solution are very high, and the chromic acid concentration is also very high. Provided that there is enough evaporation from the chromium plating tank, the washdown solution can be returned to the plating tank, but purification to first remove metallic impurities is recommended. Ion exchange is one method, but the life of the resin is short.
One-time use of the resin is a possibility. One liter of resin will remove about 2 equivalents of metal impurities. Unfortunately, trivalent chromium is also removed and it would be better to oxidize the trivalent chromium back to hexavalent before purification, or to use a membrane cell to re-oxidize and also remove impurities.
consultant - Cleveland Heights, Ohio
Do other plating solutions use PFOS/PFAS wetters & fume suppressants like chrome plating?
Q. I am an environmental consultant, looking into the use of PFOS/PFAS-containing fume suppressants in the metal finishing industry. I have 2 questions:
1) I am aware that foam blankets are used in chrome plating and anodizing. But are the same foams used in nickel, zinc, gold, copper, or other plating operations? Or other types of foams? and
2) I understand some other plating uses a "wetter" to alter surface tension and reduce bubble size. Isn't this effectively the same goal as a foam blanket? They are all surfactants, so might the "wetter" also have PFOS/PFAS?
- Denver, Colorado
October 16, 2020
A. Hi Bill. While I don't claim that other plating solutions are mother's milk, the extreme environmental issues we talk about in chrome plating are pretty much restricted to chrome plating for a number of reasons.
• Hard chrome plating / engineering chrome plating tends to be orders of magnitude thicker than other platings -- often being used for such purposes as rebuilding worn components, and requiring far higher currents.
• Chrome plating is horribly inefficient. Only somewhere between 10 and 25% of the applied electricity deposits chromium, and all the rest generates hydrogen gas. Other plating solutions are usually 75-99.9% efficient, generating way less hydrogen gas. If you reduce the plating current in other solutions they just plate proportionally slower, but chrome plating simply stops below a threshold current.
• Strong chromic acid is an extremely powerful oxidizing agent which precludes the use of most surfactants, whereas the surfactants used in most other plating solutions are sodium lauryl sulphate or similar compounds -- essentially baby shampoo.
To my knowledge PFOS/PFAS is not and was not used for any solutions other than chromic acid.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Need photo of chrome tank with mist suppressant please
![]() Thanks, Ted. Very helpful information.
Thanks, Ted. Very helpful information.
Would anyone on this forum be willing to send me a good (well lit, good resolution) photograph of their chrome plating mist suppressant foam for a summary article I'm writing for environmental folks who know little about plating? If someone was so inclined, please frame the photo to dominantly or entirely show the liquid surface, like this one: https://www.researchgate.net/profile/Per_Moller2/publication/268350210/figure/fig1/AS:383566182666240@1468460712993/1-1-An-industrial-hard-chrome-plating-system-The-sample-to-be-deposited-with-chromium_W640.jpg.
And I'd be happy to attribute the photo to you as in "Image courtesy of Harry Helpful, Generic Plating and Finishing, 2020, used with permission." I need something that is not already copyrighted by someone else that you'd allow me to use for this article.
Email is [deleted by editor in 2023 for privacy since no longer needed]
Very much appreciate any help anyone can provide.
- Denver, Colorado
November 3, 2020
Q. Good morning sir.
During chrome plating we face issues/details below:
1) Fume Small (Please suggest chemical name for controlling fumes).
2) Plating cracks after grinding,
3) pin holes,
4) poor plating.
5) Bleeding in nose
Plant Head - PUNE India
August 12, 2021
by Fasya & Lusno

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Bhoopendra. Please search the site for threads about topics 2, 3, and 4. We have about 100 threads on those chrome plating topics. Obviously you are doing one or more things wrong, but until you tell us what you are doing via a pretty complete data dump, it's hard to answer.
Topic 5 is a very serious problem, to which Topic 1 is an answer. Other vendors can offer their equivalents, but Atotech and their "Fumetrol" products have been mentioned.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. How to control the emission rate >0.006, i.e., fumes in chrome plant? To control it the consultant of our company tells us to use Fumetrol 140, and before adding it to find the surface tension, i.e., to find the surface tension of the tank solution and to buy tensiometer. My question: is there any other way without buying this Fumetrol 140?
Jiyaul haqProduction engg.(chrome plant) - Chennai& tamilnadu, India
August 2, 2023
A. Hi Jiyaul
There are four ways that I can quickly think of to reduce airborne chrome emissions:
1). Use a fume suppressant -- an oily skin on the surface of the tank, which captures much of that mist.
2). Use a surface tension reducer, which limits the ability of the little droplets to form around the hydrogen gas and be carried out as bubbles.
3). Reduce the solution level in the plating tank so a greater percentage of the mists simply fall back in rather than escape to the ambient air or an exhaust system. I once visited a plating shop with exceptionally deep plating tanks designed for plating older very long battleship cannons. For the newer, shorter, barrels, they lowered the solution level in the tanks to about 36- 42" inches below the tank rim, and the reduction in emissions was incredible.
4). Employ an exhaust system with dry mesh pads or fume scrubber to capture the escaping mists.
Focusing first on the first point: fume suppressants have been very successful but Fumetrol 140 is PFOS-based. PFOS is a 'forever chemical' which environmental regulators have been focused on for a while, and which the public around the world is now extremely concerned about. Although I don't know the regulations in Chennai, I do not think you should use it anywhere in the world here in 2023.
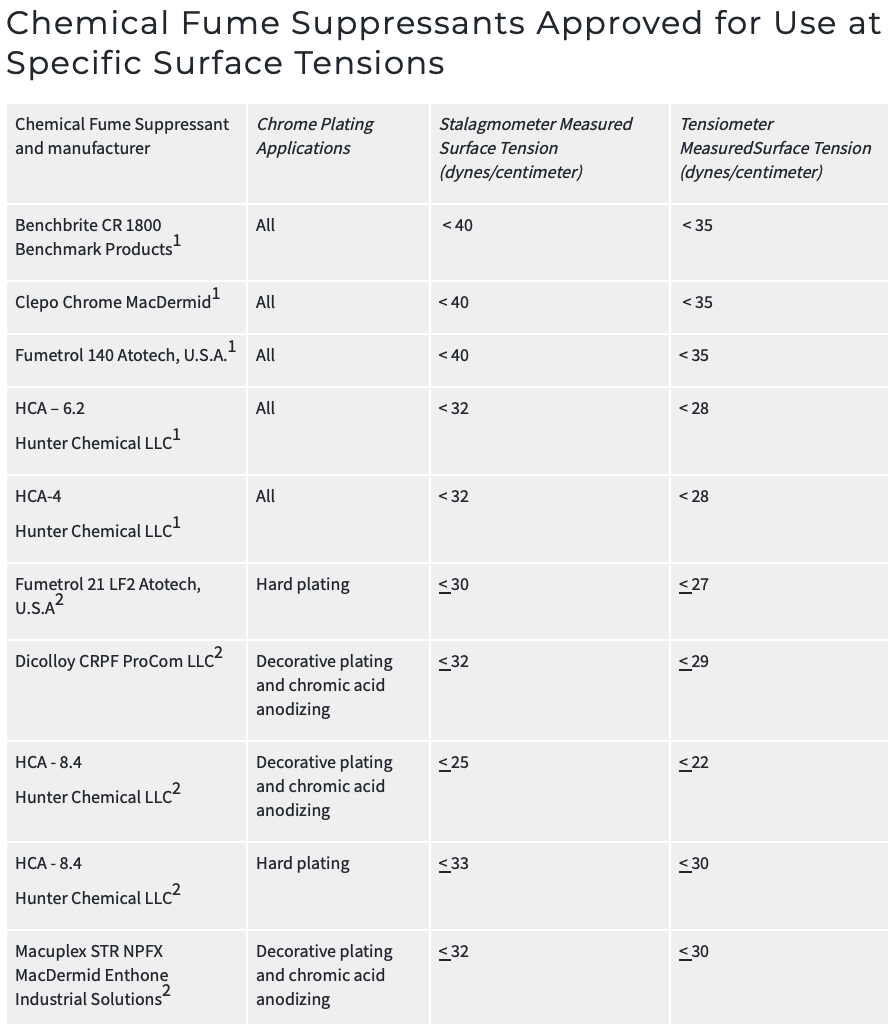
Non-PFOS fume suppressants are, to my limited understanding, available but not as effective ... which brings us to the second point: a combination of surface tension reducer plus non-PFOS fume suppressant may do the job. This graphic from the California Air Resources Board gives a quick flavor for the issue ⇨
If you are able to employ even a relatively small measure of point three, along with one and two, that would be great.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Dear Sirs,
I'm requesting info about the type of tensiometer that we should use in hard chromium platings baths: Noüye Ring or Withelmy Plate.
Thanks in advance
SHOP EMPLOYEE - BARRANQUILLA, COLOMBIA, SOUTH AMERICA
October 6, 2023
Q, A, or Comment on THIS thread -or- Start a NEW Thread