
-----
DIY Copper Plating as student experiment or a hobby
Q. I'm a high school student doing an experiment on the effect of epsom salt [affil link] on electroplating. I want to plate copper onto zinc. I'm using a 6-volt lantern battery. The copper strip is connected to the + side and zinc is connected to the - side. The solution I'm using is 10 grams Cupric sulphate diluted in 100 mL of water w/ 2 grams of salt added. I keep getting a black tarnish on the zinc and a more build up of black stuff when I added the salt. I have tried different types of zinc materials like zinc plated and galvanized cathode but I still got the same result. What am I doing wrong?
Mary [last name deleted for privacy by Editor]student - Houston, Texas, US
2004
A. Hi Mary. Please start with our FAQ on "Electroplating: How it Works". Consider either electroplating copper onto nickel or brass, or plating zinc onto copper. You can't properly plate electroplate copper onto zinc from simple salt solutions like yours because those metals form a battery and the copper spontaneously deposits with no adhesion. Also, please use only 1-1/2 volts, 6 V is way too much and will create many problems. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
2003
Q. Would I get good results from using a regular vinegar ⇦in bulk on eBay or Amazon [affil link] plating bath for copper. I have a steel piece to flash prior to silver plating.
Thanks,
Fred Readhobbyist - Duluth, Minnesota USA
2005
A. No, Fred. Sorry. Copper plating of steel requires cyanide plating solutions or proprietary pyrophosphate plating solutions. The vinegar stuff is strictly for demonstration of the principle for science projects, and even then the students apply it to more noble metals rather than steel. You can nickel plate directly onto steel though, then copper if desired, then silver plate. Best of luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
2005
A. No. Acidic copper baths will form immersion deposits on steel and adhesion will be poor. Since you're silver plating, why not just use a silver strike and forget the copper?

Jeffrey Holmes, CEF
Spartanburg, South Carolina
2005
2005
Q. Dear Sir,
I would like to ask some questions:
1. what is the best formula for copper before it's being nickel chromed?
2. how to clean the things / the subject before it is copper plated?
Thank you for the solution
Regards,
Rudy
hobbyist - Jakarta, Indonesia
A. Hello, Rudy. Your questions may sound reasonably specific to you, but they are actually demanding of some detailed tutoring in fundamentals not easily achievable in a forum like this.
1. There are many different general formulations for copper plating: acid copper plating like copper sulphate ⇦ on eBay or Amazon [affil link] and copper fluoborate; more neutral solutions like copper pyrophosphate; and cyanide copper plating. Each has applications: for example, pyrophosphate is applicable for plating on steel without using cyanide, whereas acid copper won't work. Cyanide copper works well on diecastings or on aluminum which has received a zincate pretreatment, but the others don't. Acid copper gives harder brighter deposits. But in any case, most people do not make their own copper plating solutions, they buy the solutions with proprietary addition agents which improve the plating.
2. The cleaning depends on what the substrate is made of; but if it is steel, then alkaline electrocleaning would be the usual mass production method, whereas scrubbing with pumice and detergent might be a good hobbyist method. After the cleaning, the object requires activation by dipping in acid.
Please see our "must have" booklist and consider trying to get your hands on a good plating text or two. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
2005
September 7, 2015
I need to do copper electroplating on 0.5 cm^2 gold substrate. How could be this done?
I need minimum roughness also.
How could I treat the electroplated copper (can I rinse with DI water after the experiment)?
Could you please help me in this...
- Bangalore,KARNATAKA, India
September 2015
Hi Krishnadas. I believe a sulphate-based bright acid copper plating solution should work and that your project sounds reasonably easy (just make sure the gold is polished before you copper plate it). I am guessing that you are at least at undergraduate level, but you haven't introduced us to your situation -- and not knowing whether you're a grade-schooler or a post-graduate makes it hard to offer reasonable advice. Try to get a copy of the Metal Finishing Guidebook. This will introduce you to the various copper plating solutions and the general aspects of electroplating. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Thanks for your kind attention. I am a Phd student in Mechanical Engg. I need to have 10 micron Cu height over 100nm gold to make a channel for liquid flow. The roughness should be minimum in order to have a smooth flow of liquid across the channel.
Thats where I am stuck.
Could you please help me with this?
- Bangalore,KARNATAKA, India
September 8, 2015
A. Hi again. A picture is worth a thousand words. This is an open top channel? You are trying to build vertical copper walls of 10 micron height but any convenient width onto a gold surface? That would be done by masking the gold where you don't want the copper, and then copper plating the rest to a thickness of 10 micron. If the masking is smooth, the copper plated up against it can be smooth. See some similar work:
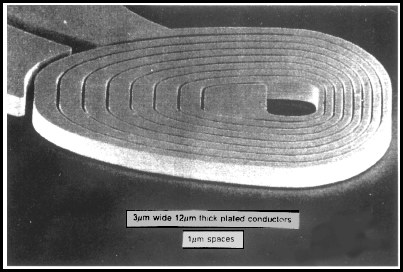
from Electrochimica Acta, Vol. 42, nos 20---- by L.T. Romankiw
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
September 2015
Q, A, or Comment on THIS thread -or- Start a NEW Thread
