
-----
Titanium plating (Electrodeposition OF titanium)
< Prev. page (You're on the last page of the thread)
by Donald Mattox

on eBay or Amazon
or AbeBooks
(affil link)
Q. Currently we are trying to deposit Ti over SS by molten salt electrodeposition method, up to now we didn't get any positive result. Can anybody suggest the process parameters like selection of salt, current, temp.... Hoping for some reply.
Haran [surname deleted for privacy by Editor]ME student - Coimbatore, Tamil Nadu, India
2004
Q. Not many water soluble Ti compounds out there. Make a new one. Isn't there Ti in bones? If it's in your body, isn't it water soluble?
Anthony Brown- Dayton, Ohio
2004
A. Hi Anthony. There's more to it than dissolving titanium into a water soluble compound. You still have the issue of reducing it before the hydrogen in the water reduces.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2005
Q. Electroplating of titanium from sulfuric acid solution. In reference to plating Titanium, why must it be reduced before hydrogen in order to be plated? Please explain.
Margaret
engineer - San Diego California
November 23, 2009
A. Hi, Margaret. Let's assume you have dissolved some titanium into the sulfuric acid, so the solution contains titanium ions. For simplicity we'll say they are Ti++ ions, although titanium can have other oxidation states too.
To electroplate the titanium as a metal onto a substrate we need to add electrons to those ions to reduce their oxidation state from
+2 ionic state to the 0 metallic state:
Ti++ + 2e- => Ti0
But if (which is the case), this reaction is much much "easier":
H2O + 2e- => H0 + OH-,
Then what happens is that you can't electroplate titanium out of an aqueous solution because the electricity you put in all just goes to liberating hydrogen from the water. The same goes for aluminum and some other metals.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Ever tried dissolving standard liquid paper in acidic solution and then plating the residue titanium byproduct? (hint, hint)
Ti02 can be dissolved in hydrochloric acid, chlorine gas is released and Ti is left in H20 solution, reduce and restore pH as necessary adding dissolved rock salt saline solution, and then plate titanium as per copper sulphate
⇦ on
eBay or
Amazon [affil link] solution,
About 95% of titanium ore extracted from the Earth is destined for refinement into titanium dioxide
⇦ on
eBay or
Amazon [affil link]
(TiO2), an intensely white permanent pigment used in paints, paper, toothpaste, and plastics. It is also used in cement, in gemstones, as an optical opacifier in paper, and a strengthening agent in graphite composite fishing rods and golf clubs.
When used in the production or handling of chlorine, care must be taken to use titanium only in locations where it will not be exposed to dry chlorine gas which can result in a titanium/chlorine fire. A fire hazard exists even when titanium is used in wet chlorine due to possible unexpected drying brought about by extreme weather conditions.
alchemists intuitory - Hoppers Crossing Victoria Australia
April 12, 2010
A. Hi, Daniel. Yes, you can obtain titanium compounds and dissolve them in acid. But, no, you can't plate them out of an aqueous solution like you do with copper. The electrons you supply to the cathode will not reduce the titanium ions to titanium metal, but will simply liberate hydrogen gas from the water. The same reason you can't electroplate (from an aqueous bath) with magnesium and aluminum.
Based on some study of the history of chromium plating involving proven fictions in Russian reports from a particular time period, these Russian reports of titanium plating from the same time period would appear to me to be a fiction. Guessing is no substitute for experimenting, but I'm not equipped to do the experiment, and we haven't yet heard from anyone who has done it. Thanks. But take a look at Brenner first, which notes that finding titanium in the deposit doesn't necessarily mean you have deposited metallic titanium, it may be an occluded titanium compound. In fact, the popular Ti-Cad plating is designed to do exactly that: occlude a small amount of titanium compounds into the cadmium plating.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 13, 2010
Q. I am researching depositing small layers of Ti metal, I have found a reference that describes depositing Ti at room temp out of organic salts:
Electrodeposition of Ti from TiCl4 in the ionic liquid 1-methyl-3-butyl-imidazolium bis (trifluoro methyl sulfone) imide at room temp: study on phase formation by in situ electrochemical scanning tunneling microscopy
I. Mukhopadhyay et al Electrochemica Acta 50 2005 1275-1281
My problem is I'm looking to deposit on the order of 50-500nm and this paper shows on the order of Angstroms shown with STM! If any of you take a look at this let me know if you think that I can deposit this relatively large thickness from what they show.
Thanks,
Ed
- Columbus, Ohio
2005
A. A granted patent in China and Taiwan and pending patent with USA, Japan, Australia and EUs of a process of depositing advance materials such as titanium under atmospheric pressure has been developed which is favorable for thin film coating.
The process requires further R and D to turn it to practical use. Published detail of US pending patent application no. 10/130,582. The writer is the sole applicant and ownership of IP.
- Hong Kong
2006
Q. Titanium plating fairly thick (150 microns) layers on a polished glass substrate, releasable, is of interest for fabricating the thin mirrors needed for astronomy in space. Nickel mirrors are successfully produced in this way, particularly by Media-Lario in Italy, but titanium may be better in terms of weight and stiffness.
For our Luciola project of stellar interferometer (www.oamp.fr/lise/seminaires/LabeyrieCNESLuciola.pdf) we need 100 or so mirrors, 200 mm in size, having a modest optical quality since they serve as small solar sails.
Any test results, with electroplating or other methods ( Schoop projection , etc...), will be highly welcome.
College de France - Caussols, France
2006
Q. So after all, did anyone try Mr. Bujian's plating recipes?
I am about to try a process for depositing titanium by CVD, using the tetraiodide under flowing hydrogen at 450 °C. Does anyone have experience with this process? From what I've found,a DC or RF plasma is necessary, and thermal CVD will be insufficient. Can anyone confirm that?
manufacturing - Newton, Massachusetts
2006
A. There are many Russian books with chapter on titanium plating. Anybody can download some of them from next website http://lib.prometey.org/ (if you can read Russian). Hope it helps and good luck!
Goran Budija- Cerovski vrh Croatia
March 2, 2010
Q. I am also very much interested to plate titanium onto a metallic surface from an electrolyte solution and that too at around the room temperature. Are there any such processes existing ? Further - has anyone ever tried out the co-deposition of titanium and boron to get titanium di-boride plating? For that matter, is co-deposition at all possible from mixture of salts ?
thanks
- Kharagpur, West Bengal, India
2006
A. In reply to Asimava Roy Choudhury. Titanium diboride can be plated from molten salts - please check out Journal of The Electrochemical Society, 156 (2009) D131-D137 where it was deposited from NaCl-KCl-NaF-K2TiF6-KBF4 at 700°C
Adam Whiteheadelectrochemical surface technology - Wiener Neustadt, Austria
January 14, 2010
|
A. As an alternative to water, I believe the solution may be reduced to a white/clear powder and then mixed with certain molten semi conductor without hydrogen, hey seriously if y'all are worried about people counterfeiting coins I will stop posting (hint hint) Alchemists Intuitory - Hoppers Crossing Victoria April 13, 2010
 Ted Mooney, P.E. Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for instant help or longer-term assistance. April 14, 2010 |
February 6, 2011
Q. Here's my problem: I have a unit on my swimming pool that produces chlorine by electrolysis of salt water. The unit obviously consists of an electronic control unit and a cell containing anode / cathode plates through which water is pumped. I have had the unit for about 5 years and it has worked perfectly up until now. The electronics is still OK, the electrolysis cell has given up. I tried to contact the original manufacturer but they've gone belly up.
According to the original sales literature the plates are titanium coated, which makes sense considering the reason for the electrolysis - i.e. to liberate chlorine not electroplate the electrodes.
The original cell is a sealed unit but I have managed to open it and modify the case to allow removal of the plates. The plates appear to be mild steel with a black coating which I guess is titanium. From the years of continuous use the plates have corroded especially around the connections used to carry the electric current, which have corroded away.
Now the questions: Is there a way I can coat mild steel plates with titanium at home? From what I've read here electroplating is out of the question. If not would someone let me know where I can have some of these plates manufactured. Or what about titanium plates, would the cost be astronomical? Finally, if titanium plates are an option is it possible to weld onto titanium, the original plates have 4 mm rods spot welded to form the terminals?
- Johannesburg, Gauteng, South Africa
A. Hi, Roy. Titanium is more costly than steel, but less costly than silver. It requires heliarc welding like aluminum (see www.thefabricator.com/article/arcwelding/titanium-you-can-weld-it). It is available as solid sheets, and as a mesh, and probably as a "boxed mesh" like aluminum screens for windows.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 7, 2011
A. Hello Ted, nobody get your point. I appreciate your efforts. The patents mentioned in this thread are still pending though it is irrelevant. We have to revisit the theories that were postulated and accepted as common sense until now to change our viewpoints over reactivity, especially to solve this issue.
Thanks and Regards
- Bhubaneswar, India
November 5, 2012
A. Hi everyone,
Ted is kind enough to respond in a polite manner to every comment and his arguments are more then reasonable. Sometimes nature puts up some walls that we cannot cross. This is simple nature, the Ti is more active than H2, and you cannot plate it from water.
I did my PhD in Chemistry and often I had to read scientific literature, and I can tell you that is difficult to reproduce the chemical reaction published in the majority of journals, no matter if there are Russian, or European or American; so do not hope too much from this. This is because, often, the authors do not give all the tricks, and also because it can go on mg scale but not on g or kg scales. Also, from my experience, a lot of Russian literature is very questionable.
If there is no commercial solution to titanium plating, means that it cannot be done. It's that simple! CVD or PVD is another topic that have nothing to do with the real plating.
Cheers,
- Barcelona, Spain
May 31, 2013
A. Hi Bajaji. Thanks. I understand that titanium can be electroplated from ionic liquids, and I suspect that we'll have a revolution in the electroplating industry one day soon as we move from aqueous solutions to ionic solutions!
![]() Thanks for the kind words as well as the technical input, Radu!
Thanks for the kind words as well as the technical input, Radu!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 31, 2013
A. Titanium plating patent pending:
www.metalfinishing.com/view/24839/an-alternative-approach-to-plating-of-titanium-and-ti-alloys-using-carbon-foam-substrate/
- Oceanside, California, US
September 15, 2013
A. I am planning to work on electrodepositing Titanium from a simple salt bath onto a given substrate.
The fact that Titanium is more negative than hydrogen does not mean that Ti could not be electro or electrolessly deposited from aqueous solution of simple or complex salt bath onto a substrate. I am looking for answers to this challenge.

Bassey J. Udofot
Musgegon, Michigan
November 30, 2013
A. I dare say the answer is most probably yes!
Though it's not necessarily simple... As most stated correctly the metal is highly "active"... Which means the reduction potential is very negative. That doesn't mean BY ANY MEANS that it cannot be done. What that means is that it cannot be done with water as solvent.
So, if you are hell bent on doing it, it's just a matter of finding a suitable non-aqueous medium. I'd suggest try doing it in pyridine medium first since there is a high chance of success.
- Rio de Janeiro, Rio de Janeiro, Brazil
August 8, 2014

Feb. 25, 2022: Sorry that domain (crista.com) seems to no longer exist
I am excited that I am working with a company that has discovered the electroplating method for Titanium and I am also a big part of that process.
Atul Gupta- San Diego, California
April 2, 2015
Q. Hello:
If I had TiCl3 in aqueous solution and I heat it to 425 °C Ti salt melting point) at that point I could perform electroplating, only relying in Ti3+ and Cl- ? ... or is it a silly question?
Many thanks!
construction - santa maria del cami, mallorca
June 7, 2016
Hi Victor. "Aqueous" means water-based. Water boils and vaporizes at 100 °C, so the problem is: how can you heat an aqueous bath to 425 °C.
But yes, I believe it is possible to plate some titanium out of a fused salt bath ... it's probably a very long trip from electrodepositing some titanium in a laboratory setting to doing practical electrodeposition of titanium. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
June 2016
A. In this patent they affirm that they are able of produce a Ti coating by electrodeposition from H2SO4.
https://patents.google.com/patent/US9023187B2/en
- San Sebastian, basque country, SPAIN
April 6, 2017
![]() I know this sounds crazy, but I may have "accidentally" electroless plated a nickel-titanium alloy onto borosilicate glass. I was EN plating at my company this morning and thought I would do a little impromptu "R&D", if you want to call it that. I heated 300 mL of mid-phos "hybrid-proprietary" EN solution to 194 °F in a 500 mL beaker [beakers on
eBay
or
Amazon [affil link] and added 3.00g of titanium boride powder, followed at once by 20 mL of 35% hydrogen peroxide solution, in an attempt to raise the -ide to -ate, and hopefully prevent it from fighting with hypophos for Ni+ reduction rights. Apparently only a tiny volume of the titanium salt actually dissolved, but it seemed to catalyze the deposition of Ni-Ti onto the inside of the glass beaker. Stripping the deposit with concentrated HNO3 revealed that the coating was about 60/40 Ni/Ti, respectively. Naturally, the Ni portion was stripped fairly rapidly by nitric, leaving the Ti portion to "fall off" on its own, afterwards.
I know this sounds crazy, but I may have "accidentally" electroless plated a nickel-titanium alloy onto borosilicate glass. I was EN plating at my company this morning and thought I would do a little impromptu "R&D", if you want to call it that. I heated 300 mL of mid-phos "hybrid-proprietary" EN solution to 194 °F in a 500 mL beaker [beakers on
eBay
or
Amazon [affil link] and added 3.00g of titanium boride powder, followed at once by 20 mL of 35% hydrogen peroxide solution, in an attempt to raise the -ide to -ate, and hopefully prevent it from fighting with hypophos for Ni+ reduction rights. Apparently only a tiny volume of the titanium salt actually dissolved, but it seemed to catalyze the deposition of Ni-Ti onto the inside of the glass beaker. Stripping the deposit with concentrated HNO3 revealed that the coating was about 60/40 Ni/Ti, respectively. Naturally, the Ni portion was stripped fairly rapidly by nitric, leaving the Ti portion to "fall off" on its own, afterwards.
Anyway...I'm going to follow up on this experiment in a more quantitative method as soon as I get a chance. Since my curiosity is now triggered, it may not be too long.

Randall Fowler - Fowler Industrial Plating, LLC
Cleveland, Tennessee, USA
May 10, 2017
? Dear Mr. Fowler,
I am curious to know whether you were finally successful in plating Ti or not?
- Tehran, Iran
October 5, 2017
Hello Behrouz bahadormanesh,
I have only had the opportunity to try this procedure one other time, with similar results. I have not yet been able to establish a step procedure for it. I apologize for not being more active with it, but keeping my company running has taken front seat. I have all of the salts and equipment ready to go, so I should be able to get back to it shortly after the first of the year.
Thank you.

Randall Fowler - Fowler Industrial Plating, LLC
Cleveland, Tennessee, USA
December 14, 2017
Q. Hi, I have been looking into titanium plating for a bit and I have found a research paper where they managed to create a surface of titanium-iron alloy by by using molten salt, not sure if anyone has found it before but it may be useful to some.
http://www.tandfonline.com/doi/abs/10.1016/j.stam.2006.02.016
Basically I want to get an anodised finish on mild steel but steel itself can't be anodised, I want anodising mainly for aesthetics as similar levels corrosion protection can be achieved with electroplating other metals alone, however I'm trying to find a metal that can be plated onto and then be anodised, is there any metal that can be plated and then anodised?
Just as a note the metal for plating doesn't have to be titanium, it's just the one I was looking into at this time, anything that can be plated to steel using fairly accessible home methods and can be anodised after that will do.
Also would it be possible to alloy titanium to some other metal like nickel that could make it easier for the titanium to be plated, or would it just split the alloy created and just leave the titanium behind?
Cheers :)
- England, Lancashire, Rossendale
June 15, 2017
Q. Want to put a titanium finish on a polished bronze sculpture, is this possible?
Thank you,
Charles gibilterra Design - Carmel, California
December 4, 2017
A. According to Russian sources it is possible to plate titanium from water based solutions!
http://www.galvanicrus.ru/qa/?answer=302
(galvanicrus.ru is leading Russian webpage dedicated to electroplating and finishing!)
- Cerovski vrh Croatia
February 10, 2018
Ed. note: Readers can plug that URL into translate.google.com to see it in English
A. Another possible solution:
www.materialstoday.com/metal-finishing/features/an-alternative-approach-to-plating-of-titanium/
- Austin, Texas USA
February 12, 2018
April 15, 2018
A. Yes. Ti can be plated in a water bath. Here is my recipe:
Ti(NO)4 0.06 gr
TiO2 4 gr (anastase)
HNO3 50% 25 gr
ZnO 0.08 gr (pore filler)
Sodium metabisulfite 4 gr (chemical reducer)
water DI 500 ml (chlorine generates H2)
DC 3.25 V, 20 A, on low carbon Fe cathode works fine; graphite anode, filter paper bag. Agitate. T = 21°C. Minimum 30 minutes.
Dorecol Ltda. - Medellín, Antioquia, Colombia
Q. I am responding to the last thread dated April 15,2018 posted by Jorge Velasouez L from Colombia.
For the sake of transparency, I would request the author to share the data supporting his claim that he was successful in electroplating Ti using this recipe in aqueous solutions. Any SEM-EDX data, cross-sections, thickness validation?
- MECHANICSBURG, Pennsylvania, USA
March 7, 2019
Q. This is in reference to Goran Budija's post about three recipes in a Russian book. I would interested to see the actual data obtained with any of these recipes. Any published papers by the unknown authors? While attempting to plate Ti in aqueous media, hydrogen evolution overtakes the Ti deposition rendering almost zero current efficiency w.r.t. Ti deposition.
I have tried first two recipes and unfortunately those do not work. I am sorry that erroneous statements or citations in plating literature do not help the plating community. Honesty and transparency should be exercised in reporting scientific results.
Dr. S. LAL
- Mechanicsburg Pennsylvania USA
July 26, 2020
![]() Hi Dr. Lal,
Thanks for trying them. Most of we participants in this forum have always believed deposition of titanium from an aqueous solution to not be possible for the reason you mention.
Hi Dr. Lal,
Thanks for trying them. Most of we participants in this forum have always believed deposition of titanium from an aqueous solution to not be possible for the reason you mention.
Yes, unfortunately there are a good number of wild claims in old Russian technical literature, sometimes not due to intent to deceive but analytical limitations.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
July 2020
February 11, 2021
A. 2 old USSR patents on titanium plating...




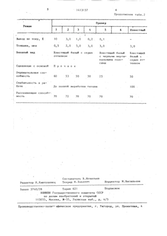
(click thumbnails to see full size)
- Zagreb Croatia
Q, A, or Comment on THIS thread -or- Start a NEW Thread