
-----
Watts Nickel Plating Problem: pH is too low. Why? How to Fix?
Quickstart:
Various cations/cation radicals can be used for nickel plating, i,e,, the plating solution can be nickel chloride, nickel sulfate, nickel sulfamate, nickel fluoborate, etc. Each has advantages and disadvantages. The most commonly used nickel solution for decorative plating is Watts Nickel -- a solution of nickel sulfate, nickel chloride, and boric acid.
For Watts nickel plating to proceed properly the pH must be correct and the solution must be buffered with boric acid.
This thread addresses mostly pH going too low. If your problem is the opposite, please see thread 97/50, "Watts Nickel Plating Problem: pH is too high. Why? How to Fix?"
![]()
⇩ Related postings, oldest first ⇩
Q. Our plating bath has low pH for the last couple of weeks. The Nickel pellets were activated in sulfamic acid for a day (and rinsed) before they were changed out. That was our 1st try to raise the pH. Dummy plating doesn't seem to help, nor does running production. I've seen many resources stating that Nickel Carbonate will raise pH. What I haven't found is by how much. All I've seen are generalities like "some" or "just a little".
My question is what is "just a little"? 5 grams, 100 grams?
Does it depend on volume? Is there a easy formula that states "To raise pH .1, add X Nickel Carbonate?
I understand it is best added as a slurry.
By the way, it's been 2.6-2.8 where it had consistently been at 4.0. Any help on the addition would be great.
Jeff Dingmanan electroforming company - Rochester, New York
2003
A. Nickel carbonate is not very soluble. Do not add it to a nickel plating tank directly. The best way is to make a slurry of water and nickel carbonate and recirculate it into a filter. Transfer the filter hoses into the nickel tank, turn on some agitation and run the filter until the pH reaches the value you want. Remove the filter, clean it and use it for continued filtration of the nickel tank. I prefer to use nickel oxide in the same way as above. It has less impurities than nickel carbonate and dissolves easier, but it's possibly more difficult to locate a source for small amounts. If the pH of your nickel plating solution is going down while plating it is an indication of insoluble anodes, or not enough anode area (anode CD too high causing polarization). A normal operation of nickel plating solution requires the addition of acid to maintain the pH.

Don Baudrand
Consultant - Poulsbo, Washington
(Don is co-author of "Plating on Plastics" [on Amazon or AbeBooks affil links]
and "Plating ABS Plastics" [on Amazon or eBay or AbeBooks affil links])
A. The amount of nickel carbonate will depend on its purity, the pH level and the tank volume. However, nickel carbonate is a potential carcinogen and personally I do not like using it; I prefer altering the pH with sodium hydroxide. I appreciate the increase in sodium ions is supposed to increase stress, but I have not knowingly experienced it. If you really want to use nickel carbonate, make it into a slurry and slowly add it to a by-pass tank and gently agitate the tank until it has all dissolved. It is very insoluble and prone to "glob up" if you are not careful; it will also effervesce vigorously, so wear a respiratory mask. Monitor the pH as you add the carbonate, but allow the tank about 10 minutes to "relax" before taking the pH again - it is like a piece of good roasting meat! You can take the batch bath pH up quite high before anything significant happens and once it is returned to the bulk tank the extra acid will be consumed by any hydroxide that is formed in the treatment tank. The real trick to keeping the pH under control is to keep the bath under control. It looks like you are using a sulphamate bath, so in theory you should be able to control the pH by having adequate boric acid (at least 30 g/l and preferably 35-40) and, more importantly, to have the correct nickel anodes. You should use sulfur depolarised nickel and these will dissolve in a sulphamate bath without the addition of chloride. However, most platers prefer to add about 10 g/l nickel chloride "just in case" (This is their very own comfort blanket!). If you do not use SDP nickel, you must use nickel chloride -- I would suggest about 30 g/l -- but be aware this will adversely affect your stress. The anodic reaction will thus be primarily the dissolution of nickel and not the discharge of oxygen, which results in the decreased pH.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
A. Indeed, double check your Nickel Anode pellet material. Pure Nickel pellets are for Chloride strike baths, and SDP pellets, a common type are known as "S-Rounds" are for sulphate & sulfamate build baths. Pure Nickel pellets in a sulfamate bath won't corrode at all therefore the only reaction available at the anode will be water hydrolysis yielding sulfamic acid & oxygen...causing pH to drop. Even with S-Rounds if this acid formation reaction is still a pest you can shut it down by adding a bit of NiBr anode corrodant. With proper anodes, Boric acid content, and a bit of NiBr anode corroder you should be fine. The Ni Carbonate works excellently, but you will have to experiment a bit with beakers to determine your own dosage chart because you will be adding it to a buffered system. But I think you'll need to wait a lot longer than the aforementioned 10 minutes for equilibrium! I used to wait sometimes up to 2 hours for pH stability.
If your anode area is just too small due to space restrictions and they continue to throw off a lot of acid, you can still help them out a bit by flushing them with plating solution during plating. This requires specialized anode assemblies plumbed directly in line with the filters.

Dave Kinghorn
Chemical Engineer
SUNNYvale, California
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. The pH is low in my Nickel plating tank. I've used hydrogen peroxide to pull it up a bit but really need to add some Nickel Carbonate. Is either Nickel Oxide or Hydrogen Peroxide a good alternative?
Steve Clarkplater - Belfast, Maine, U.S.A.
2004
A. I would not think hydrogen peroxide is ideal, Steve. But in the thread which we appended your inquiry to, Don Baudrand says yes to the nickel oxide.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Steve, I am guessing that you purchased the old West Plating outfit in Belfast. Been awhile since I lived in Maine (1986) I was that plater in Camden. Did a lot of stove parts for Bea Bryant in Thorndike. Anyway. Since you probably have limited access to chemical and do not wish to spend a ton on Nickel Carbonate or Oxide, I suggest using NaOH DILUTE! (AR grade the best) With lots and lots of agitation. It is a long process as you do not wish to overshoot. Make sure your Boric acid is where it should be and remember it is a buffer so as you add the Caustic you will suddenly get a jump in pH. Calibrate your meter well or make sure your pH papers are fresh. You will need to filter well and I suggest Dummy Plating prior to use. Test a part and look for roughness on the shelf. Bigger question: why is pH so low? How's your anodes and bags? Did you just overshoot on a downward adjustment? Can't say I haven't done that more than a dozen times.
Jon Quirt- Minneapolis, Minnesota
Q. Overshot on downward adjustment due to lack of information. pH is slowly rising and still plating well -- just can't plate any aluminum at the moment. Wouldn't the sodium be a contaminant? I've got sodium hydroxide on hand, and don't mind taking my time.
Steve Clarkplating - Belfast, Maine, U.S.A.
A. Steve,
Sorry for slow response. I would not worry about the Sodium. If pH is coming back on line just hold that course. Your efficiency will be lower so plating times more. Stress could be an issue but since yours is decorative just keep an eye on it. Hard way to make a buck isn't it?
Jon Quirt- Minneapolis, Minnesota
Q. Dear Sir,
How could one raise the ph of nickel sulphate bath of ph 2.68 to 3.8-4.2. We have just re-constituted our bath and noticed that the pH is 2.68. When we electroplated on this bath we noticed that we cannot separate the grown plate from the mother plate. I will very grateful for any useful suggestion.
⇐ answer?
Nigeria security Printing & Minting Company- Lagos, Nigeria
2006
Q. Our plating solution is 130 liters. We adjust our pH for 4.2, and after one day of working our pH is reaching 3.8 or 3.6. Please help me how to eliminate this problem.
Saeid Ghasemzadehengineer - Tehran, Iran
2007
A. If the pH of a Watts type nickel is decreasing instead of the normal rise I would suspect that you have clogged anode bags or empty anode baskets which are causing hydrogenoxygengas to evolve.
process supplier - Great Neck, New York
![]() Hi. I believe that our always astute Mr. Packman made a little typo. The plating solution, when unable to dissolve nickel at the anodes for the reasons he mentioned, evolves oxygen gas. Oxygen evolving from the water leaves the solution with excess hydrogen ion, causing the dropping pH.
Hi. I believe that our always astute Mr. Packman made a little typo. The plating solution, when unable to dissolve nickel at the anodes for the reasons he mentioned, evolves oxygen gas. Oxygen evolving from the water leaves the solution with excess hydrogen ion, causing the dropping pH.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Dear Friend,
When you pass current, hydrogen evolves from cathode and oxygen evolves from anode. Since nickel is a high efficiency bath the decrease of hydrogen or increase of pH will be lower. So it is recommended to go for a buffer salt of around 45 gpl, normally boric acid. Then check the pH once in a hour or so and add some acid to maintain the pH.
Regards
- Aurangabad, Maharastra, India
A. Hi friend,
This is common. Please ensure pH before reducing. Normally pH should adjust with vigorous agitation. Anyway there is no problem. Actually pH 3.8 is good for nickel plating bath. You can continue the production. Simultaneously pH will rise, or else you reduce the anode area for one day production. This will increase pH of the bath in good manner.
Don't try to add caustic soda ⇦liquid caustic soda in bulk on
Amazon [affil link]
to raise pH. It will create a lot of problems in the future.
GOOD LUCK.
- Bangalore, Karnataka, India
A. Just another note to consider. Be sure you are not dragging in any acid to the bath, or low ph rinse (from stagnant rinses).
Mark BakerProcess Engineer - Syracuse, New York
![]() Dear All,
Dear All,
I completely agree with Mr Baker.
That could be the exact reason.
Regards
- Aurangabad India
Q. The amount of nickel carbonate needed to raise pH of a Watt's Nickel Bath will depend on the purity of the nickel carbonate, the pH level and the tank volume. It seems most people say to make a slurry. Is there a formula I can use?
Debra Cellettilab manager - East Providence Rhode Island
April 19, 2010
A. Hi Debra
Dave Kinghorn offer good advice on this subject.
Obviously your anodes are not dissolving correctly causing you to experience pH dropping.
To find out how much NiCO3 YOU need, do a lab test of taking say 1 liter in a container and adding weighed small amounts of NiCO3 to find out how much is needed for your particular circumstance. Take note of how long it takes for the reaction to complete.
Each situation is different so you need to experiment to find out what you need, not some theoretical amount.

Geoffrey Whitelaw
- Port Melbourne, Australia
Q. Previous month, we encounter high dog-bone. Plating deposited higher on the edges. We tried to increase the Boric acid concentration from around 35 g/L to 50 g/L. After doing this, the dog-bone returns to normal level. Sorry I'm not so expert in plating, could anyone explain this theoretically (and is it probably one of the factors)?
Thank you
- Cavite Philippines
May 24, 2023
A. Hi JM. Boric acid content is one of many things that may help limit "dog-boning"
The others being chiefly the primary current distribution, but secondarily the solution agitation, the plating speed, and specialty ingredients (brighteners, carriers, levelers).
Higher boric acid levels, as long as the do not lead to it precipitating out, are probably good so I am not going to try to dissuade you if you feel you have some evidence that supports the process working better at 50 g/l than 35 g/l.
As for what the boric acid does: it is a buffer, but it doesn't buffer the pH of the plating bath as a whole because the bath operates at an acid pH, and boric acid doesn't do any buffering function at that pH. Rather, within the boundary layer, right at the point where the plating actually takes place, the pH tends to want to jump to 9 or more, at which point the nickel would precipitate out. Although I don't pretend to truly & fully understand the rich dynamics of exactly what is happening at the atomic level, that is when the buffering action of the boric acid kicks in and keeps the nickel from precipitating on the surface.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Thank you Sir Ted. I'm relieved that the action conducted is OK.
As continuation, we already applying 50 g/L and concentration is ranging from 44 g/L to 50 g/L. Yet we are still encountering increasing dogbone (at this concentration range). But during additional of Boric acid to target 50 g/L or just adding Boric Acid, dogbone is gradually going down and maintain to normal level.
I'm planning to add Boric Acid Daily to maintain 50 g/L (or to target 50 g/L)... I'm just worried of the possible effect of doing such in the future.. What do you think ?
Thank you in advance.
- Cavite Philippines
May 26, 2023
A. Hi again, JM. If you are operating a proprietary nickel plating bath, you really should get your advice from the seller. But if it's home-brew, 30-45 g/l is the usual range for boric acid; but if 50 g/l seems to discernibly reduce dog-boning, again, I can't see a good reason to not operate that way.
The only issue I see is that if you let the bath cool down to ambient temperature you are at about the solubility limit and might get some boric acid precipitating out which would have to be thoroughly re-dissolved.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. If you increase the boric you must also increase temperature.
Dull nickel does not mean no additive.
You need to purchase from suppliers.

Sara Michaeli
Tel-Aviv-Yafo, Israel
June 21, 2023
Thanks for additional input. Temperature of the solution is set to 50 °C +/- 5 °C.. Currently not encountering any problems from appearance, precipitation, etc..
Boric Acid concentration is already reaching 50 g/L to 55 g/L
- Cavite Philippines
June 26, 2023
Q. Hi JM, Hi Ted!
Could you explain what dog-boning is?
Perhaps it's a translation issue, but I've never heard of it.
I'm imagining the shape of a bone, so would that mean higher deposition at the corners of the parts due to a lack of micro throwing power?
JM, are you directly depositing nickel onto your parts or do you have copper before this layer?
- Nova Friburgo, Rio de Janeiro, Brasil
July 3, 2023
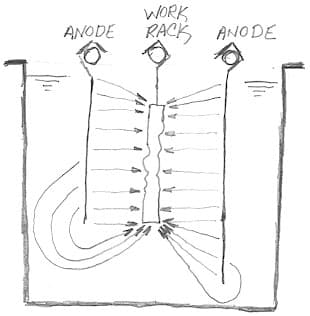
A. Yes, Pedro, more deposition at the corners and/or the ends of the piece than in the middle.
In JM's case, boric acid content or micro throwing power might have something to do with the problem, but a common cause of dog boning is simply poor anode sizing or placement. Remember that Faraday's Law tells us that plating thickness is directly proportional to current, and that current tends to take the path of least resistance, so if the anodes are as long or longer than the work, dog boning is a surety :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Hello Pedro, Ted gave some great advice and I just wanted to add what I have experienced with dog boning. In the Printed Circuit Board industry dog boning was common until plated holes got so small and multilayer boards got so thick, acid copper baths and current density had to be adjusted for compensation. High acid, lower metal content, solution carriers, good cathode bar and solution agitation, lower current density applied with anode to cathode ratio of 2:1. With working with 10 mil holes and board thicknesses of .125" we could get surface to hole ratios of 1 to 1.2.
If you make sure your work is lower than the anodes as Ted points out, you will want to look at things like getting a full chemical analysis, rack and solution movement improvements and possibly a current density adjustment. This would go for other plating, not just copper. Good Luck and hope this helps.
- Cazenovia, New York USA
July 10, 2023
Q, A, or Comment on THIS thread -or- Start a NEW Thread


