
-----
Sulfamate Nickel Strike Problems
Current comment:
A. John,
Your copper needs to be cleaned, before you can sulfamate strike, also we do run our temperature at 130 degrees, our immersion time is 3.5 minutes.
The blisters are from the product not clean. Dirt under blisters.
Line operator, working with sulfamate strike a lot - south bend, Indiana
May 2, 2022
⇩ Related postings, oldest first ⇩
Q. We are using the Barrett Nickel Sulfamate Strike process to plate a two circuit SSt-copper laminant panel. We run 75 g/L Ni, 30 g/L boric, pH 1.2-1.5 and [Cl-]=1.4% by volume. Temperature is 30 degrees C (86 F) and our plating time is 2.5 to 5 minutes. We get good adhesion of the strike to the SSt and copper circuit of the panel. The other circuit on the panel is copper only and we DO NOT plate it with the strike (but it does get exposed to the chemistry for the entire strike time). We then follow up the strike bath with a quick spray rinse and a nickel sulfamate plate. The problem is that we get blistering on the copper that does NOT get nickel strike. Anybody have any ideas what is possibly going on here? Our metallic contamination of the strike bath is very low (< 2 ppm in Cu and Cr).
John Hutchison- Eau Claire, Wisconsin
2002
|
|
2002 A. Obviously, the strike bath is deleteriously affecting the unplated copper; the question is how or why. My suspicion would probably be that the unplated areas are becoming bipolar anodes such that they are partly nickel plated under poor conditions on one side and partly made anodic and passive on the other side. You could run some parts through the strike bath with the current off to see if my wild guess has validity, or if mere exposure to the solution is the problem. In the worst case you'd have to mask the unplated area; in the best case your rack could have some simple shield pieces that would stop the bipolar action. Just a wild, sight-unseen guess :-)  Ted Mooney, P.E. Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for quick help or longer-term assistance.
A. Dick Crane or Jim Will should be able to handle this problem. Meanwhile, here is a long shot. If the Barrett Strike is very old, then at that pH most of the sulfamate radical has broken down into ammonia ⇦ on eBay or Amazon [affil link] and sulphate and the sulphate is attaching to the uncharged bare copper, Perhaps a mechanical wipe with Scotch Brite and another hit electrolytically this time in the strike might prepare the surface to receive plating without blistering. OR, make up a 5 gallon brand new strike and see if the same thing happens. This will confirm my theory about the breakdown into sulphate.  Robert H Probert Robert H Probert Technical Services Garner, North Carolina  2002 |
Q. More questions for Ted's response:
1) If it is a bipolar effect (Cu-only circuit acting as an anode while the SSt-Cu Laminant circuit is the cathode) why don't we see similar blistering when we expose the parts to a standard sulfamate nickel bath? Does pH have an influence on the bipolar effect? What is the anodic reaction of the copper that would cause poor adhesion?
2) Does it make sense to lower the current density to minimize the bipolar effect?
3) Earlier testing at higher current densities showed that we would get a very thin deposit of Ni on the copper-only circuit ... even though it was isolated from the SSt-Cu laminant circuit.
Thanks,
John Hutchison- Eau Claire, Wisconsin
2002
A. John, the bipolar effect I'm speaking of is not the same thing as a galvanic effect; it doesn't depend on the difference in materials, it depends on their orientation between the anode and the cathode, as shown in the exaggerated picture below:
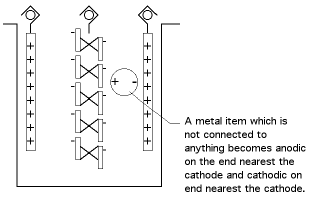
The bipolar effect in your case would be much less, but can account for the nickel you see deposited on the copper. A portion of the copper which is not connected to anything will be anodic, and will have a small amount of copper dissolving from them; and a portion will be cathodic and will have nickel depositing on them.
The effect is probably more dependent on voltage applied than on pH.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for quick help
or longer-term assistance.
2002
Q, A, or Comment on THIS thread -or- Start a NEW Thread

