
-----
Barrel plating dangler problems and alternatives
Quickstart:
Readers who are unfamiliar with 'barrel electroplating' may wish to start with --
"Barrel Plating, by Frank R Zemo"
Long parts (screws) zinc plating
Q. Hi everyone,
We are experiencing some issues in our zinc barrel plating process with long parts (8" screws as the photo). The parts are bending in the process with 2~3% bent parts as average (some much more than the one in the photo) even with slow rotation and doing all the process carefully.

Has anyone experienced this type of deformation in the process? I heard of some other type of processing as in-between from barrel and rack (some sort of oscillating open barrel instead of a rotating closed one).
I would appreciate if anyone can share what you know on this matter :)
Thanks a lot, have a nice day!
- Cañuelas, Buenos Aires, Argentina
October 26, 2023
A. Hi Daniel. If you're pretty sure that the bending is happening while the screws are in the plating barrel rather than in material handling, I can think of two approaches:
1. Danglers must snake their way through the parts as the barrels rotate, and this can cause snarls of parts, which then bend. Plating barrel manufacturers offer barrel styles of contact mechanisms which do not have danglers. They tend to be more complicated and require more maintenance because they use a sort of contact brush system within the hanger arm so there is nothing stationary within the barrel for the load to twist up with.
2. Some years ago, manufacturers offered doorless barrels to facilitate automation; I think they called them "rack & roll". In order that the parts not fall out during plating, the barrels only rotated 180°or so before reversing. You would not need to go doorless, but could simply install a timing mechanism and reversing contactor to reverse the rotation of all your existing barrel drives, so they rock back and forth 180° to 270° and the danglers don't wind up.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Hi Ted, and thanks for your insight here.
Hi Ted, and thanks for your insight here.
We make those screws from scratch, heat treat them and zinc plate them, so we know for sure they are bending inside the barrel while plating.
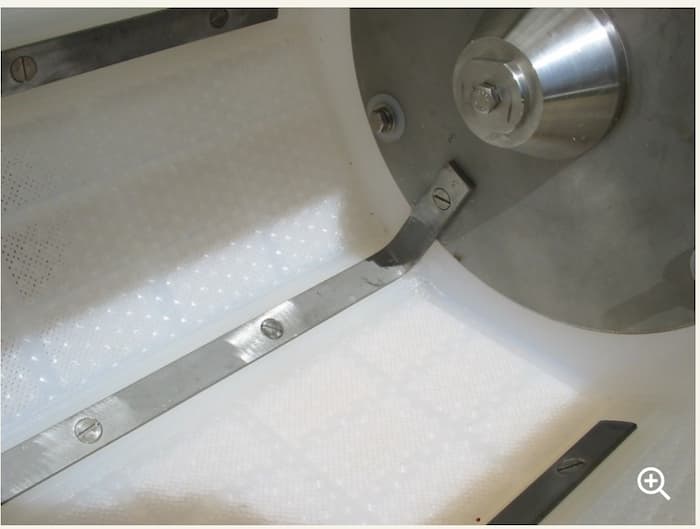
Now we are using a bar and triangle cathode system in this particular zinc plating line (we have another one with danglers, it's not suitable for long parts like these). As they are case hardened and very sharp pointed, they damage the barrel as the photo. There you can see the cathode system and some damage in the PP barrel.

I have heard of this open barrel system, but here in Argentina we don't have this technology available. We can have it made, but those products aren't very high volume so we don't expect it's worth the investment.
YES we will try to lower more the rotating speed and try to reverse the rotation every two rotations or so, but as this zinc plating line has the rotating motors and gears connected for 4~7 tanks (the barrels don't have the motors, the rotation triggers by gears down in the machine and in the barrel), we don't know if it will make the belt motor slip or if the motor will be able to make this motion.
So, I'll be in touch when we can try this so my experience can help our fellow platers here.
Thanks again, see you around.
- Cañuelas, Buenos Aires, Argentina
November 21, 2023
A. Hi again Daniel.
Yes, barrel contacts of that general sort rather than with danglers is what I was speaking of. Sorry that it didn't help enough.
Since the plating barrels have doors and won't spill, it shouldn't matter if several are ganged off the same motor or starter. Maybe just try a timer arrangement where you rotate for 6-10 seconds in one direction, stop for a second or two, then rotate 6-10 seconds in the opposite direction, and repeat. Ideally, the motors should be duty-rated to insure they can do this without overheating, but If I were you I would try it anyway :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇩ Related postings, oldest first ⇩
Q. I have a problem Zinc plating a hook on a barrel line. My barrels are 36" x 24" and are rated at 500 Lbs. capacity, but these hooks catch against each other, against the dangler, and bend each other out of shape. I've had to reduce the rpm's to 2 rpm and I am only able to load the barrels to about 80 lbs.

What should I be plating these hooks in? Vibratory barrels? Please help.
Thanks again,
Rodrigo Salinas- El Paso, Texas
2002
A. Most "vibratory" barrels are employed on electronic parts 25 times smaller than yours.
Parts tangling together is a perennial problem in barrel plating and there are only partial solutions, which include: different contact styles (cones, bars, external buttons, etc.) in lieu of danglers, small loads (like you are running), and periodic reversing starters so the barrels only rotate a few seconds in each direction.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Vibratory barrels may be a good option, but they are expensive. May I suggest trying a barrel with button danglers, and also using conductive plating media or some other zinc plated parts that can be mixed with the hooks and separated later.
Karl Weyermann- Lebanon, Kentucky, USA
Q. OK, vibratory barrels are too expensive & too small, so let's talk about button contact barrels, cone contact, or other forms of dangler-less system. Does anybody have a good diagram on how to retrofit a regular barrel?
Rodrigo Salinas [returning]- El Paso, Texas, USA
A. A common reason a dangler snarls up when plating nesting parts is that the dangler does not rotate with the barrel and the mass of parts; it must keep snaking its way through the mass. So, the hope is that a style of contact that rotates with the barrel--so there is nothing stationary in the revolving barrel--will minimize the snarling.
So, what you are trying to do is to get the electricity to the parts without leaving anything stationary like a dangler inside the barrel or mass of parts. The buttons, or contact bars, or cones, or whatever, get fixed to the cylinder and rotate with it and the parts.
It is a bit tricky to do yourself because it requires some kind of informal "electrical brushes" arrangement whereby those contact surfaces fixed inside the barrel get their current from a contact on the hanger arm. But just picture if the hub on the barrel's hanger arm were made of metal instead of plastic, and so too was the bearing sleeve on the barrel, so metal would be rubbing on metal instead of plastic on plastic, with the metal hub on the barrel cabled to the rectifier, and the metal sleeve on the barrel connected to whatever contacts you choose. That's exactly the idea you're trying to accomplish while minimizing the metal's exposure to plating solution. Plating barrel manufacturers are able to supply such contact arrangements for dangler-free contacts though. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Just read the thread.
Just some ideas for barrel design to solve issues.
(Novo Metal Finishing Equipment "Cone Strip plating barrel")
Director, KUHU INDUSTRIES PVT LTD - jaipur
September 12, 2025
A. Hi Rodrigo Salinas,
If you're looking for a long term solution for plating these hooks I would suggest you to go for rack plating. I guess you can expect uniform deposition and faster plating rate. It's possible to rack and unrack these hooks quickly.
Venkat Raja- Kitchener, Ontario, Canada
Can we get Dangler knobs that are passivated
Q. We do barrel-electroplating (Nickel, Copper, etc.) We use steel danglers in barrels (not St. Steel) From time to time we remove plated deposit from danglers to reuse dangler again. We remove coating by hammering them.
Questions:
1) Is there a way to passivate the steel dangler so that removing the deposit becomes very easy?
2) Is there an easy way to remove the deposit instead of hammering the danglers?
Thanks a lot for replying to these questions
Pravin AagashePlating Supervisor - Bombay, Maharashtra State, India
2003
A. Hi Pravin. You could try electropolished stainless steel dangler knobs. Electropolished parts have very little "tooth" and are quite passive, so it's generally easier to remove deposits from them. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Why not Titanium dangler knobs?
Q. Hi
Why is titanium never used as a dangler in barrel plating of decorative finishes?
Obviously it is more expensive than copper or brass from the outset, but the copper & brass danglers need constant cleaning & attention to remove the plating that accumulates on them. Would it not be less work using titanium danglers?
buyer - Durban, KwaZulu-Natal, South Africa
2007
A. Titanium is a terrible conductor of current, so it would not work as a dangler.

Jon Barrows, MSF, EHSSC
Kansas City
Q. Hi Jon
Thanks for your reply.titanium of course works as a conductor when used as an anode basket for nickel chips.
Surely, then., it would be acceptable as a dangler?
- Durban, KwaZulu-Natal, South Africa
A. Natasha,
As Jon mentioned Ti is not used for danglers because of lower conductivity and higher contact resistance. Copper is the 2nd best conductor, I think brass is the 6th best, at room temp of course. As you said Ti is more expensive. Based on these two facts alone, what kind of contacts would you put on danglers? Ti is used for hooks and baskets mainly for its strength, heat and corrosion resistance, it is compatible in certain plating solutions.
process Engineer - Syracuse, New York
A. Hi. There's a bit of a semantics issue here. The dangler as a whole is a heavy copper cable with heavy plastic insulation and a bare contact knob on the end. I'm sure Natasha is speaking only of making the knob out of titanium, not the whole cable.
I could be wrong but I think the actual problem is not conductivity or wear, but polarity. My belief based on practical experience is that titanium is not actually resistant to the acids used in plating: rather, the titanium oxide coating it develops from being anodic is what is acid resistant. Dangler knobs are cathodic and I think they will quickly dissolve in the plating baths.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Are Copper Danglers F006 Hazardous Waste?
Q. Why are used danglers classified as F006 waste? With copper over $3.00 a pound why can't they be sent to a recycler instead of my company having to pay to get them hauled off as F006. We send them off 300 pounds at a time. Thanks
Mike CappsLab Tech - Bon Aqua, Tennessee, USA
2006
A. Who decided that they are hazardous waste, Mike?
I don't think you're interpreting a regulation correctly. Although I'd need to restudy it myself (can you give us a reference?), but I don't see why you can't strip the rubber off, triple rinse it and dispose of that part as simple waste and sell the copper as scrap.
What reasoning are you using, or others giving you, to cause you to equate a dangler with a sludge? Clearly the components that you plate aren't hazardous; why would the danglers that you plate be considered hazardous?
In the case of a disputed interpretation, common sense should prevail; it seems to be an environmental travesty and an assault on sustainability to landfill copper! At the very least, anyone who issues an order to do so should sign their name to it :-)

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Barrel leads (danglers) break when plating long fasteners
Q. Dear sir,
When we plate long length fastener items like length M10x210 mm or M10x238 mm, barrel leads break during process and we have to face loss of time, and cost.
Is there any way to stop such type of problem?
Help me!

Kapil Gupta
tools - Faridabad, India
May 22, 2011
A. Dear Gupta,
Use a tight PVC sleeve over the solder Joining point (Dangler and the cable).
All the best.
- Bangalore, Karnataka, India
Q. We now use a Solder Stripper to periodically (every four hours!) clean the Tin off the cathode buttons in my plating barrels. This chemical is my highest quantity of hazardous waste generated and I have made it one of my goals to reduce or eliminate. Any suggestions along those lines would be helpful. The chemical is Ammonium Bifluoride and Hydrogen Peroxide, so it is a safety concern as well as a hazardous waste.
My idea is to periodically take out the anode basket, but in a blank panel and then reverse the current to plate off of the buttons (as anodes). Is this feasible? Can it be done? I worry about plating off the Chrome or Iron when they are clear of Tin. I could make the buttons out of Platinized Titanium or Zirconium if that was a concern, but I feel that could become a wearing issue as well.
Thanks for any thoughts on this.
Mike
Department Engineer - Lake Mills, Wisconsin, USA
March 11, 2013
A. Hi Mike. Just brainstorming here, but I am not yet convinced that plain button contacts wouldn't work. They could be made of electropolished 316 stainless steel, which is both very passive and very free of tooth; I think with luck the plating would fall off or almost fall off.
As for deplating, I don't think you will attack the substrate of the buttons if you keep the voltage low.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Thanks Ted,
Let me clarify. The cathode buttons are Stainless Steel. They are slightly attacked by the stripping solution now, so they do not stay smooth long, but become dull. Normally, they are bright (decorative) but they are not SS316 either, probably SS304. (We use platinized Titanium for anode parts.) The Tin plated onto the cathode buttons forms a granular surface, very tenacious and also plates out onto the PE barrel surface, all within about 4 hours of use. The total current is 23 Amps at about 7 Volts DC.
- Lake Mills, Wisconsin, USA
A. Why not use a nitric acid stripper formulation instead of ammonium bifluoride? It would not attack the stainless steel. Other than the fumes, it may have less concerns about human exposure.
Lyle Kirmanconsultant - Cleveland Heights, Ohio
|
|
Q. The company had used a Nitric acid based stripper in the past. Fumes were an issue then. I agree that using an oxidizing acid would be the way to go. I will see if using a more dilute solution might still work without the brown fumes.:) - Lake Mills, Wisconsin, USA A. Another route, avoiding attack on the stainless steel, would be to dissolve the tin electrolytically in sodium hydroxide.  Harry Parkes - Birmingham, UK |
Substitute for copper rod in plating barrel
Q. Hello
I use barrels to coat mild steel (MS) spokes with zinc; I use acid zinc process. The copper rod used to provide current to MS spokes (cathode) catches a lot of zinc deposit. can I substitute copper with some other material, perhaps titanium?( I have tried copper wire but the wear and tear is fast)
- mzn India
October 15, 2020
A. Hi Sam. Can you send a photo to mooney@finishing.com for posting here? I'm not sure what you mean by "the copper rod". Most plating barrels have danglers. But some plating barrels have, instead, a copper rod running from the center of one hub to the center of the other, from which you can dangle something to bring current to the parts. But making a suggestion how to improve what you have when I don't really know what you have is tough :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello,
I'm struggling with plating (zinc) off long workpieces (nail-screw-spike) 30-35-40 Cm /11-13-15 inches)
Due to production volume process have to be carried in barrel system. I want to avoid bending of product and entanglement.
That's why I'm looking for solution related to construction of plating barrel.
I'll appreciate your help and tips .
- Bydgoszcz Kujawsko pomorskie, Poland
December 22, 2020
A. Hi Åukasz,
I think regular dangler contacts would be best, but that the barrels should be 11" internal diameter or less so there is no way the spikes can get vertical but must essentially remain horizontal.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread