
-----
Measuring silver plating thickness
RFQ: I am looking for suppliers of coating thickness gages with a representative in Mexico.
Alejandro Martinez Vazquez- Mexico
1998
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
RFQ: Dear Sirs,
Please advice if your company has instrument able to measure the application of silver (conductive) on copper (non-ferrous). If yes please indicate if destructive or non destructive test and quote one unit including air fright to our address Cairo/ Egypt
Regards,
Alshater Tawfekengineering and scientific systems. - Cairo Egypt
2000
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
Seeking Instrument to measure silver plating thickness on copper
Q. I am looking for an instrument that will measure the silver plating thickness on a .059 copper wire. The plating thickness is .005" and the form is a spring
Thanks,
Kimberly Wentworth2001
A. I would recommend x-ray Fluorescence.
Jeff Vernier- Mckinney, Texas
2001
A. Are you sure your plating is 0.005" thick and not 0.0005" thick? At 0.005" that is 5 mils thick. I doubt that X-ray fluorescence can measure silver that thick on your part. It is true that on small diameter wire XRF is the best method but not at 5 mils of Ag thickness. Can you please verify this?
Chris Horvath- Chicago, Illinois, U.S.A.
2001
Measuring silver plating thickness on copper
Q. I have a requirement for measuring the thickness of silver electrodeposits on copper. The plated components range from 40 to 60 inches in diameter. The testing will be performed on the production floor and in the field. Therefore the equipment needs to be somewhat portable, non-destructive and non-contacting. The silver thickness is three thousand microinches (0.003 inches). There may be an 100 microinches of nickel between the silver and copper in some cases. I currently have beta-backscatter and XRF equipment.
I would appreciate any suggestions on performing this type of testing.
Barry W [last name deleted for privacy by Editor]- Blacksburg, Virginia
2003
Q. Hi, I am looking for plating thickness tester which should be non destructive. I have to measure silver plating on brass, aluminium and other copper alloys - should be capable to test on the curved surface. If you have information please let me know.
Vibaker M- Bangalore, India
May 22, 2010
A. Expensive - but XRF can do this quickly and easily after calibration using pieces of known thickness. Then you could measure dozens of pieces per hour nondestructively.
Terry Tomt- Auburn, Washington
Measure thickness of silver plating on busbar
Q. Dear Sir,
I work in switch gear factory and we commonly used copper bus bars of different sizes i.e. 10 x 100 x 4000 M in length and cut to our order sizes.
Can you name the type instruments used to measure thickness of silver plating in MICRONS on copper bus bars ?
Regards
ARSALAN
QEHS MANAGER - KARACHI, SINDH, PAKISTAN
February 23, 2011
A. Xray fluorimeter would do a fine job. Kind of expensive though.

Trent Kaufman
electroplater - Galva, Illinois
A. An X-Ray thickness tester certainly can be used. However, a destructive method can also be used, like the Kocour Electronic thickness tester. You may be able to cut a section of the plated bar for Quality Control Checking.

Ed Budman [dec]
- Pennsylvania
With deep sadness we advise that our good friend Ed passed away Nov. 24, 2018
July 7, 2014
Q. I am looking for a way (equipment) to measure the thickness of Silver (Ag) 7-12 microns, and Silver/Silver Chloride (Ag/AgCl) 9-14 microns for pads on flex circuit. Do you have any suggestions? Cross section is not working due to the softness of the Ag material.
Steve Bognacki- Syracuse, New York USA
? Steve,
May I know flex circuit is which substrate (Aluminium, Copper or its alloy, Steel, Zinc alloy, etc.)?
Process Engineer - Tumkur , Karnataka , INDIA.
July 8, 2014
July 2014
A. Hi Surya. Unless I am misunderstanding, Steve is talking about a flexible circuit board which is built on flexible plastic, usually mylar or teflon.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
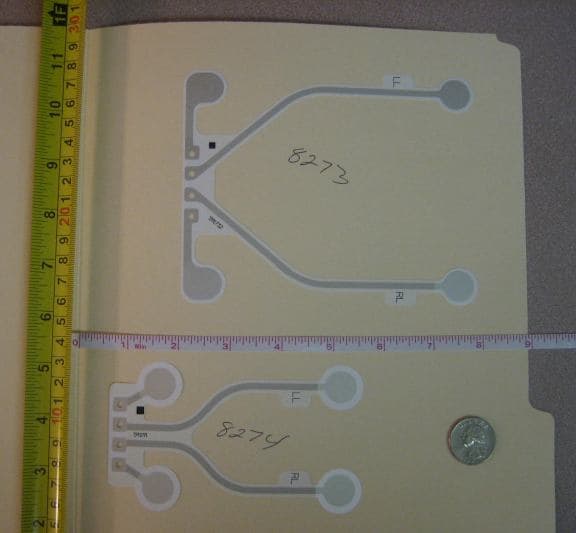 July 10, 2014
July 10, 2014Q. Materials:
Carrier: .005 (0.13mm)thick polyester film, white
(The circuits are shown sitting on a plain manilla folder not related to the problem)
Base Coat: Dielectric Ink
Conductors: Ag ink on traces total thickness 7-12 microns, Ag/AgCl ink on pads total thickness from 9-14 microns
I need a way to measure the thickness of the Ag, and Ag/Cl, but not X-Ray (too expensive).
- Syracuse, New York USA
Can't Measure Thin Silver Coating on Copper with Mb-tube XRF
Q. Hi,
I'm using an XRF with moly tube which has sensitivity issues with Silver coating on copper. When there is significant silver coating, I'm able to measure accurately and when coating 3 µm or less, the readings or not so accurate. But the same sample, If tested with an W tube XRF, it measures even a flash silver coating accurately.
My question is what actually causes moly tube to become sensitive to measure silver or tin coatings ?
Request an answer at the earliest.
Application Support - Mumbai, India
October 5, 2016
Ed. note -- Readers: Please offer generic/technology suggestions rather than specific vendors (why?)
Q, A, or Comment on THIS thread -or- Start a NEW Thread
