
-----
Plating of ABS Plastics; palladium and electroless nickel issues
Dear Raafat Albendary: Sir, I want to electroless nickel plate on polyester resin statues. But every 2 statues my bath has decomposed. I want to know about EN bath composition.
sameer sheikh- Delhi, India
April 19, 2021
A. Dear sir,
Your name is very familiar to me. For many years one respectable gentleman worked with me -- hope you are him.
For plating of polyester with palladium method, some precautions should be considered:
1 - polyester is usually filled with calcium carbonate which increases the pH of the solutions especially Pd sol'n. So it precipitates causing decreasing acidity.
2 - because of insufficient activation, platers tend to increase the reducing agent which is the main factor of instability.
3 - also they tend to increase the temperature, adding another reason of instability.
So if you are him please advise; in my next response I will tell you about a very economical and direct method to plate resin even if it's filled with calcium carbonate.
Regards
Raafat
- RIYADH saudi arabia
Multiple threads merged: please forgive chronology errors and repetition 🙂
I am researching into the bonding of electroless nickel plate to polymer coatings, and have failed to find any references - even to polymer - metal(any!) bonding. If any one out there has any suggestions of literature/web pages, etc. it would be a great help.
Thanks
by G. G. Gawrilov

on eBay or Amazon
or AbeBooks
(affil link)
The University of Edinburgh
1996
A. G.G. Gawrilov's little book, Chemical (Electroless) Nickel-Plating ⇨
Portcullis Press, Redhill, 1979, Queensway House, 2 Queensway, Redhill, Surrey, RH1 1QS. In English, ISBN 0 86108023 8 is big on content, with references from around the world. See chapter 13, Chemical Nickel-Plating in Relation to the Carrier Material.

Tom Pullizzi
Falls Township, Pennsylvania
A. Hi Monica,
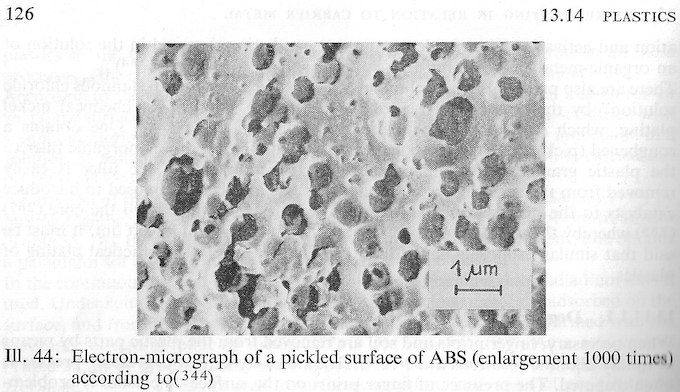
As you apparently know, plating is relatively easy, but robust plating with acceptable to excellent adhesion is relatively difficult :-)
Adhesion to plastics, unlike to metals, is not a metallurgical bond but depends greatly on the surface roughness which you able impart to the particular item, so adhesion tables may be unlikely. Bu ABS is the plastic of choice because we are able to etch it to an actual sponge-like surface, so metal can grow within the sponge for outstanding adhesion.
The book chapter which Tom referenced includes this photograph of well etched ABS ⇨
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. 1) How do we use silver nitrate 2 gms/ltr in palladium bath?
2) Our electroless nickel bath decomposes every day or two. Kindly suggest any kind of chemical which may stop the same.
Mr_Sunil_H_Jhaveriplaters - Mumbai, Maharashtra, India
2001
A. Dear sir:
1 -If you insist on using silver nitrate in activation bath:
1a -It is not recommended to put it in the palladium bath because it will precipitate as silver chloride.
1b -The formula for using silver in activation bath is: silver nitrate: 2 gm/l sodium borates: 20 gm/l.
1c -In this case you have to use electroless copper or special types of electroless nickel.
2. Instability of new E.N bath may be due to:
a -insufficient amount of sodium citrate (complexing agent)
b -over amounts of reducing agent (hypophosphite)
c -overheating the bath
d -unsuitable stabilizer (we use lead nitrate)
So you have the word now.
Best regards,
RAAFAT ALBENDARYplastics electroplating - Cairo, Egypt
? Raafat, what is your formula for an electroless nickel bath? Also, what is the cycle used to activate nonconductors for plating using silver nitrate? I have always used tin-palladium systems. I thank you in advance.
Michael Brewington- Salisbury, Maryland, USA
2001
A. Michael, I have no private formula for electroless nickel but it's my understanding that:
1-The activation metal [Ag ,Pd] it is very important to know the relation between the metal and the reducing agent in the electroless bath. Example, silver is not catalytic to the electroless nickel when hypophosphite is not catalytic to initiate or start the reaction but in borane (NaHB, Di methyl hydrogenborane [DMHB]) it will be catalytic. So the reducing agent plays a very important role.
2-The plating procedure for non conductors using silver in activation is as follows: 1-degreasing 2-etching 3-activation 4-electroless plating 5-electroplating.
Thanks and best regards,
RAAFAT ALBENDARYPLASTICS ELECTROPLATING - Cairo, EGYPT
2001
Q. Dear sir,
Is it necessary to sensitize with SnCl2 before activation with AgNO3/borate? And it will explode like fulminate of silver?
Best regards,
Prasarn Hutpattanasilp- Samutsakorn Center, Thailand
2001
A. Dear sir,
No need for sensitizing with [SnCL2] and the solution is very stable and there is no chance for explosive action.
Thanks and best regards,
RAAFAT ALBENDARYPlastics Electroplating - Cairo, Egypt
Q. Dear sir,
Do you have recommended safety procedure for using AgNO3/borate without explosion? What kind of borate used, sodium borate (sodium metaborate tetrahydrate), sodium perborate, sodium tetraborate decahydrate, sodium tetraborate anhydrous?
Best regards,
Prasarn Hutpattanasilp [returning]- Samutsakorn, Center, Thailand
by Sha, Wu, & Keong

on eBay or Amazon
or AbeBooks
(affil link)
A. Dear sir, Sodium borate is borax, which has the formula of [Na2B4O7.10H2O]. The activation cycle is simple:
1-etching
2-neutralization
3-activation in silver bath
4-rinsing [distilled water]
5-rinsing
6-electroless copper
7-electroplating
Thanks and best regards,
Raafat AlbendaryPlastics Electroplating - Cairo, Egypt
Q. Dear Raafat ,
It is sodium tetraborate decahydrate, is that right?
What type of reducing agent use in electroless copper (formaldehyde type can do this job)? And if sensitizing (SnCl2/HCl) step is add it will enhance activity?
Best regard,
Prasarn Hutpattanasilp [returning]- Samutsakorn, Center, Thailand
A. Dear sir,
1- Yes it is tetra borate deca hydrate.
2- Formaldhyde type is OK.
3- Never use HCl before any silver borate because there will be some traces of [Cl-] ions which will precipitate silver as silver chloride and that spoils the solution. And simply no need for that because [HCHO] will reduce silver [Ag+1] to [Ag0] which is able to initiate the electroless reaction.
Thanks,
RAAFAT ALBENDARY- CAIRO, EGYPT
Q. Dear Raafat,
Can you recommend the process to promote absorption of silver/borate to plastic after etching to enhance the activity in electroless copper and/or improve adhesion of metal to plastic?
Best regards,
Prasarn Hutpattanasilp [returning]- Samutsakorn, Center, Thailand
Dear sir,
I don't understand your response.
Best regards
RAAFAT ALBENDARY- Cairo, Egypt
Dear Raffat,
I mean in the conventional process when use fulminate silver as activator must use sensitizer step (SnCl2/HCl) to enhance absorption of fulminate silver (in fact reduce Ag(I) to Ag(0) ) and go to electroless copper. So if the sensitizing step is omitted the Ag species that absorbed on the plastic is Ag(1) and go to electroless copper (formaldehyde in electroless will reduce Ag(I) to Ag (0) ) so the activity will slow (covering time)? So I will ask that it has any process chemical to promote absorption instead of SnCl2/HCl or not?
Best regards,
Prasarn Hutpattanasilp [returning]- Samutsakorn, Center, Thailand
A. Dear sir, we talk too much on this subject. Why don't you try to test our claims and tell me the results.
Best regards,
RAAFAT ALBENDARYPLASTICS ELECTROPLATING - CAIRO-EGYPT
Ed. note: Agreed!
Q. Dear Raafat,
I have tried your formula and found.
1. On make up the solution goes brownish and a lot of white precipitate (maybe from commercial grade borate? We use DI water).
2. After make up the solution has brown colloidal dispersion throughout the solution.
3. The activity is so slow in electroless copper and we have skip plating.
Best regards,
Prasarn Hutpattanasilp [returning]- Samutsakorn, Center, Thailand
2001
A. Dear sir,
Try to get ready-made silver borate because what you mentioned mean that [SILVER TRACES] found in the solution when you made activation.
Thanks and best regards,
RAAFAT ALBENDARY- CAIRO, Egypt
2002
Ed. note: Raafat, your patience is legendary 🙂
Q. Dear Raafat,
I've been watching this conversation with some interest and have a couple of questions for you.
1 Does the silver activator offer any advantages over conventional tin/palladium activators?
2 How tolerant is it to different types of plating grade ABS?
3 How closely does the process have to be controlled to prevent skip plating or at the other end of the scale rack plate up?
4 How would the toxicity of this process compare to a tin/ palladium process?
Kind regards,
Tim Strickland- Auckland , New Zealand
2002
Q. DEAR RAAFAT,
I NEED YOUR HELP. WHAT IS YOUR ELECTROLESS COPPER FORMULATION. I'M WORKING ELECTROLESS NICKEL. IT CONTAINS SODIUM CITRATE, NICKEL CHLORIDE, SODIUM HYPOPHOSPITE, AMMONIUM CHLORIDE. MY ACTIVATOR IS PALLADIUM. BUT IT'S TOO EXPENSIVE. CAN I WORK SILVER NITRATE/BORANE SYSTEM WITH THIS ELECTROLESS NICKEL FORMULATION?
BEST REGARDS,
MURAT CETIN- Izmir, Turkey
2002
A. Dear friends,
Sorry for lateness.
For first friend:
what silver activator added to us:
1-it is not expensive
2-it gives us ability to plate other kinds of plastics rather than ABS [polyester-p.v.c p.s high impact] etc.
3-not sensitive to changes of pH
4-it is very stable
5-no need for sensitizing with [SnCl2]
For second friend:
My formula [actually it is not my invention] is:
copper sulphate
⇦ on
eBay or
Amazon [affil link] 5 gm/L
triethanol amin 10 gm/L
formaldehyde 20 ml/L
pH>12 [adjusted with NaOH]
temp. room
You couldn't use this activator with E.N because silver is not catalytic to nickel when hypophosphite is the reducing agent
Thanks & best regards,
RAAFAT ALBENDARYPLASTICS ELECTROPLATING - CAIRO, EGYPT
Q. Dear Raafat,
I have found interesting for your electroless copper solution. You use commercial TEA (99%) instead of Na.K.tatrate that right? TEA can be used as stabilizer?
Best regards,
Prasarn Hutpattanasilp- Samutsakorn, Center, Thailand
Q. Dear Raafat.
Thank you for your answer. Is necessary to stabilizer like Thiourea ⇦ on eBay or Amazon [affil link] , lead acetate vs? And how do you control this solution? Are you using air agitation and filtration? Have a good religious.
Best Regards,
MURAT CETIN- Izmir, Turkey
Q. Dear Raafat;
I tried your silver/borate formulation on electroless copper plating. But there is a lot of white precipitate when I was added to silver nitrate. Have you got an idea? Can we use 2 gm silver nitrate/10 ml NH3 activation system this electroless copper formulation (with SnCl2). Thanks for your interest.
Best Regards.
MURAT CETIN- Izmir,Turkey
A. Dear Murat:
If you used bi-distilled water then no problem 2-if we used silver nitrate with ammonia ⇦ on eBay or Amazon [affil link] this a very strong complex [especially with excess of ammonia] unfortunately this complex is explosive and not safe -- so get pure borate & bi-distilled water and work safely.
Thanks,
RAAFAT ALBENDARY- CAIRO, EGYPT
Q. Dear Mr. RaaFat,
Thank you very much for your answer. According to what you advised me, the ranges of PdCl2 (.007 : 5 gm/L) and SnCl2 (40 :400 gm/L) are very large, so I don't know how I could do optimal, Please explain me why the arrange of them are so large.
Thank you very much,
DAU QUANG BICH- Ho Chi Minh, Vietnam
2002
Ed. note: Finishing.com would like to thank Mr. Albendary for his yeoman work and his enormous patience, responding with ever greater detail ten times in a row!! But a well answered question yields two new questions which, if well answered, yield four more . . . and so it goes forever ...
The point of this forum is for the readers to be a mutual aid to each other, not to expect one participant to provide ever more detailed free consulting services to the rest of the world indefinitely :-)
Now is time for both the inquirers and the silent readers who have benefited from this discussion, to grab the baton and try to answer one or two of the thousands of as yet unanswered questions on this site.
Remember that when one person teaches, both people learn :-)
Q. I see your formula for electroless copper. Is it the same for all size of parts or do we need to change any composition?
Asha PatelElectroplating - Mumbai, Maharastra, India
2006
Q. I want to ask if anybody has tried the model of activator which contains silver nitrate and what was the result of that process? I want to try but firstly I wanted to ask ?
Lütfiye Canyurt- Istanbul, TURKEY
August 29, 2008
Q. Hi everybody,
After reading all this conversation I decided to use Na2B4O7.10H2O and AgNO3 instead of palladium bath. But unfortunately some precipitation occurred.
I prepared 1L bath ( 20 Na-Borax and 2 g AgNO3 )
Firstly Borax was dissolved in hot water then AgNO3, almost all Ag precipitated.
Also I tried to dissolve AgNO3 firstly but the same happened.
What is the way to get a stable solution for Ag activator?
Additionally I want to appreciate Mr Raafat for all his kindly answers over the whole conversation.
- Izmir, Turkey
November 21, 2008
Q. Dear sir,
I see mixture of SnCl2/PbCl2 is a catalyst of plastic, so I have a question, what is its purpose? Please help me.
Thank you so much.
- Da Nang City, Viet Nam
March 15, 2012
A. Hi, Ti Na.
Lead chloride is never used as a catalyst as far as I know, and it would certainly fail RoHS requirements. There was probably a typo, and you probably mean palladium chloride, but get back to us if that is not the case.
In plating on ABS plastic, the plastic is etched for good tooth, then dipped in PdCl2 or a mix of PdCl2-SnCl2 or the two in sequence. These metals are catalysts for electroless copper or electroless nickel plating solutions, so that when the plastic is subsequently put into those solutions, the Pd molecules act as seed points to begin the plating. Electroless copper and electroless nickel are auto-catalytic, so as soon as the deposition starts, it will continue.
Please hunt for a copy of ASEP's "Standards and Guidelines for Electroplated Plastics" as it is a book that tells all. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Dear Ti Na
Etching:
As you know ABS contains butadiene, which is mostly Rubber. So when it is put in acids (chromic-sulfuric) it is dissolved. Butadiene is distributed in ABS as very nice and complete spheres, so it leaves spherical holes after etching.
Activation:
Palladium & tin chloride go inside this spherical hole, but only palladium is catalytic to Electroless Ni & Cu, so we have to remove the tin and that is what they call "acceleration".
Now the palladium atom is inside the spherical hole and it is catalytic to electroless Ni & Cu, so when it attaches to electroless solution. metal starts to deposit on it so the hole will be closed with metal and start to deposit out side the hole so we can get a continuous metal layer after some time; it will be layers because of autocatalytic nature of the deposit to the electroless solutions. So now it is conductive, and we can go to electrical baths, and good adhesion since the hole is a sphere.
Hope I was clear and you got some help.
Best regards,
Raafat
National Techniecs - Cairo-Egypt BUT NOW Im Riyadh Saudi Arabia
Q. Dear Sir,
I prepared an alkaline based silver conducting solution for electroless copper according to the following composition:
- borax 20 g/l
- silver nitrate 2 g/l
When ABS articles after etching were introduced into the above solution, no silver was deposited on those articles -- what could be the reason?
Is there something missing in the above formula?
Please reply soon.
Regards,
- Elektrpkemikal, Karachhi, Pakistan
April 13, 2012
A. Dear Mr Sheikh
What we talk about is the oldest way for plating of plastic.
Mr. Ted explained one of the best and most popular methods, but now you can find direct metallization (without electroless plating) replaced with what is called (copper link); also you can find direct metallization with semi conductors method without palladium and without electroless baths too -- who knows what will happen in the next hour. My advice to you is to use this method on not smooth substrate (polyester, fiber glass, etc.), but if you insist to use this method then use silver nitrate without any added salt -- just with distilled water. Then tell me what happen. BEST WISHES
Raafat
- Egypt--but now in Riyadh, Saudi Arabia
![]() Thanks for coming back to update us, Raafat! My knowledge of this newer technology is largely "book knowledge"; I used to get out in the field all the time, but rarely do anymore. Although I've been following the subject of direct metallization in the trade press, I did not realize that it was so widely implemented already.
Thanks for coming back to update us, Raafat! My knowledge of this newer technology is largely "book knowledge"; I used to get out in the field all the time, but rarely do anymore. Although I've been following the subject of direct metallization in the trade press, I did not realize that it was so widely implemented already.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Thank you Mr Ted. It is my pleasure to be here.
Thank you Mr Ted. It is my pleasure to be here.
National Techniecs - Cairo-Egypt (but now I'm in Riyadh, Saudi Arabia)
Q. Electroless copper pH inactivity. At what pH electroless copper solution is inactive?
Naveed Sheikh- Karachi, Sindh, Pakistan
July 7, 2013
Hi Naveed. Please introduce your situation. We don't want to misunderstand your question. Thanks.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. pH is usually kept above 11 or 11.5.

Blake Kneedler
Feather Hollow Eng. - Stockton, California

avail from eBay, AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon
"Plating of Plastics with Metals" by John McDermott (1974)
avail from AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon

avail from AbeBooks, or Amazon (rarely)
"How to Electroplate Non-Metallics" by Warner Electric Co. (1967)
[more appropriate for hobbyists than for industrialists]
avail from eBay, AbeBooks, or Amazon

avail (rarely) from eBay, AbeBooks, or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
! I'm very interested in plastic electroplating.
sonu shins- Mumbai, Maharashtra , India
August 2, 2014
![]() Welcome, Sonu. Please contribute a Q or A.
Welcome, Sonu. Please contribute a Q or A.
If you are just seeking books to acquire a better understanding of the field, here are some good ones ⇨
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Electroless plating of oxide nanoparticles without Palladium
Q. Hi everybody, I'm starting to work in nickel electroless. I have nanoparticles of an mixed oxide and I want to plated them. I want to ask there is an option if we don't use PdCl2, because that is so expensive?
Lisbeth Benavides- Bariloche, Argentina
April 27, 2015
Q. We have started a manual plant 15 days back. I would like to share the layout up to Electroless nickel stage as below:
Stage 1 : Etching (Chromic + Sulfuric)-- I would like to know the best practices for composition/time/temp.
Stage 2- 6 : Water rinse
Stage 7 : Activator (Palladium Chloride + HCl)-- I would like to know the best practices for composition/time/temp.
Stage 8- 9 : Water Rinse
Stage 10 : Accelerator (HCl or H2SO4 aqueous solution)-- I would like to know the best practices for composition/time/temp.
Stage 11- 12: DM Water rinse
Stage 13 : (Very problematic) Electroless Nickel (Nickel Sulphate/Sodium hypophosphite/sodium citrate/ammonium compound)-- How to ensure the adequate chemical content? Is there any periodic testing or other method to ensure if there is adequate content in the EN bath. Is there any tank treatment required for the tank we use at EN stage. Every day, my bath decomposes or cause troubles which leads to line stoppage.
Stage 14 : Water rinse
Stage 15 : Wiping
Stage 16-Cr : Electroplating
We are fresh in this line; your guidance may lead us to get desired results.
Please provide your direction on the stages, I have mentioned above or let me know if any additional stage required.
Thanks & Regards,
- Yamunanagar, Haryana, India
May 21, 2015
A. Hi Yogesh,
I can't stress this strongly enough, but get a consultant in to help you. You are just beginning the journey and you cannot be expected to know all there is to know about electroless nickel plating.
Saying that you need to tell us the conditions you are currently using for us to be able to really help (in as much as an anonymous website can help). You need to let us know concentrations, operating times and temperatures, any potential delay times between steps etc before any sensible advice can be given.
Finally, get hold of a couple of good books on the subject and read up about the subject.
Best of luck.
Aerospace - Yeovil, Somerset, UK
Ed. note: I have been out of the consulting business for a while, but Brian is 100% right.
In most cases a consultant is not called in until several poor and costly decisions have already been made; and in many cases, the consultant can only be of limited help because some bad ideas are already locked in and cannot be rectified cost effectively :-(
At the very least call in a supplier because a simple "Woah!" from him/her can be priceless.
A. Dear Sir
1- When plastic part comes from the injection machine it has electrostatic charge; this charges adsorbs dust particles, also hand grease because of handling. All these contaminants need to be degreased so degreaser bath is needed.
2- Etching solution will dissolve the Butadiene so it will make a cavity; some chrome atoms will stick inside this
cavity so it should be removed or at least reduced to be easy to remove before activation. Otherwise it will affect the adhesion (50 gm/L of sodium bisulphite will work).
3- Before activation it's maybe better to put HCl bath in the same concentration as activation bath to precipitate or react with any impurities that will harm the activation bath.
4- I'm not understanding what is the "wiping", so please advise.
5- Reading EN bath it's better to read the beginning of this question.
Thanks & best regards
Raafat
National Techniecs - Riyadh Saudi Arabia
A. Not because I work for a supply house, but plating on plastic should not be "home brew". Palladium is too expensive for trial and error.
The supplier will also give you the required backup, plan the plating cycle (which is a long one for POP), etc.

Sara Michaeli
Tel-Aviv-Yafo, Israel
A. This is to respond to whether there is an alternative to PdCl2 (Palladium activator) --
On many research paper and patents, it has been found that a few other metals are just as good as palladium for activating the surface. The least expensive that offers the same performance is Silver nitrate (Silver chloride is insoluble in water). Silver can be used as an activating surface both as colloidal solution (about 0.5 grams per liter) after sensitizing the surface with stannous chloride, or by reducing the silver amine complex (think about silver mirrors or The Brashear process). It does not need to be a thick coat of silver -- just enough to render the surface active enough. If it takes 5 grams of silver nitrate per litter to have a good quality mirror than by using 2 instead will render the surface active enough.
For activating colloidal solution: 0.5 grams per litter of DI water. This solution should go after the stannous chloride sensitizer(5 grams of SnCl2 add 20ml of HCl per litter of DI water) rinse and rinse again, the proceed to the plating tank (the piece should never be allowed to dry).
- Managua, Nicaragua
January 14, 2016
Q, A, or Comment on THIS thread -or- Start a NEW Thread