-----
Electropolishing Nitinol nickel-titanium alloy
Q. Hi Anna, very interested in ultra polishing! Is it electropolishing processing? May you describe it? Thank you very much!
shawn xu- shanghai, China
April 20, 2022
April 27, 2022
A. Hi Shawn Xu,
This is the electropolishing process, however in certain conditions there appeared plasma on the metal surface. This plasma penetrates in between wires, thus electropolishing braided nitinol stents completely.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
⇩ Related postings, oldest first ⇩
Q. I am currently working on a process to electropolish Nitinol (Ni, Ti alloy) to a highly finished surface. If you have any suggestions regarding the chemicals and equipment I should consider using, please tell me. Thanks!
Jingli Wang1997
A. Depending on the size of your workpiece (anode) and the specifications on your rectifier, you might use phosphoric acid (quite concentrated). But beware: The current densities might be very LARGE, and you will lose weight (from your workpiece) FAST!
Regards,
Jan Morten Sraker1997
A. Jingli:
For a first information pass, you can find in the USB Library "Electroplating Engineering Handbook ⇦ this on eBay, AbeBooks, or Amazon [affil link] " by L.Durney. It has a great chapter on electropolishing various metals and gives formulations, amp/volt functions, calculations, etc. ASM Metals Handbook also has an excellent treatise. Also the Metal Finishing Guidebook is killer. These three references should be enough to get you started. Since you're in Academia I'll give you some math: "The required current density necessary to sustain a polishing condition is inversely proportional to the boundary layer thickness." You can demonstrate this on one of Dr. Newman's RDEs. I'm currently building a medium-size EP facility in Texas. I'll be happy to discuss this further if you need.
Regards,

Dave Kinghorn
Chemical Engineer
SUNNYvale, California
1997
Q. I am interested about the electropolishing, chemical polishing of Nitinol for Microstructure studies using optical microscope and scanning electron microscope.
Ganesh Kumara K.Mangalore University - Mangalore , Karnataka
1998
A. Hi ganesh
I have been trying my own luck at EP Nitinol for optical and electron microscope studies. The idea here is to get a good surface finish which can be done with a fine diamond polish.
for EP a good solution to use would be 20% H2SO4 in methanol [affil link]. You can connect it as the anode to have faster removal of the oxide and then later try to polish it at the cathode with hydrogen evolution. But you need to do a couple of trials to get your own recipe as it depends on the distance of the electrodes, surface area, current density etc.. I would suggest using low to medium voltages (around 10 V) to get a steady uniform removal. In the meantime if you have had any luck with this please do let me know as I will be interested with your progress. I have a few relevant pubs. if you are interested.
-- Guru
Guruprasad RamanathanCase Western Reserve Univ. - Cleveland, Ohio
2000
Q. Starting up a research scale electropolishing facility for laser processed metals and alloys. In particular I would like to know about electropolishing of platinum metal and nitinol (Ni:Ti) alloys.
Alan Ryder- National Centre for Laser Applications
1998
Q. I am interested in getting information about electropolishing nickel-titanium (Nitinol) tubing. What kind of cleaning & preparation would be appropriate? What polishing solution do I need, and any other info for a beginner. Can you help or direct me to help?
ed synder- san jose, California
1998
A. My experience is after electropolish you will need to do a fine diamond polish to get a mirror type finish. I was working with .032 and .045 pointed wire
Greg Townsend- Minneapolis, Minnesota
1998
Q. Since you are specializing in electropolishing Nitinol, I would like to find out about the possibility of using Nitinol as an electrode immersed in blood, but used only as a cathode. Have there been applications in cardiac or other modes of stimulation where Nitinol served as a current carrying electrode without being corroded? It is claimed to be highly bio- and hemocompatible, but what happens when current is passed through it at low densities and in pulses, rather than continuously? Does Nitinol have a protective oxide coating as titanium or is the surface similar to the bulk of the alloy?
Thank you for any info!
Peter Tarjan- Coral Gables, Florida
1998
Embrittlement from Electropolishing of Nitinol wires
My name is Emanoel R. of Almeida, and I work in a parts factory for use orthodontics. I seek information on equipment and composition of the electrolyte for polishing of Nitinol (Ni-Ti alloys). Some attempts, in laboratory equipment, presented embrittlement, probably due to nascent hydrogen. Any information is of a lot of help. Thank you advance / Best Regards.
Emanoel Ribeiro de AlmeidaSP - Brazil
1999
A. Nitinol is subjected to hydrogen embrittlement. Bake after your process for 30 minutes @ 400 F. You will be ok again.
Todd Huehn- Blaine, Minnesota
2002
Safety issues in electropolishing of nitinol
Q. Heh people,
I am a chemical engineer with a medical device company in Ireland. We are interested in the area of Nitinol stent production. This for us would have to include electropolishing. I understand that Nitinol electropolishing is a difficult thing to master and that very few people attempt it. I presume this is due to difficulties in electrolyte composition. I know of one recommended electrolyte which is composed of methanol [affil link] and sulfuric acid. To me, this seems like a dangerous arrangement of chemicals; the methanol being organic and the acid being an oxidiser.
Could anyone who has used this arrangement please inform me of relevant safety procedures they employ. Is the solution proprietary or would we have to make it up from scratch and if so, what is the method for doing this safely. Are there any other suggested electrolytes we could use? Is the solution heated?
All help is greatly appreciated.
Best regards,
John Martyn- Ireland
2001
A. In order to polish Nitinol in environmentally safe solution, you need to use special polishing equipment.
adv.
Yes, we can help you in polishing Nitinol. Please visit our home page to see some description of the process.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
2001
Q. I am encountering a surface defect related problem in some nitinol wire that I have been able to plate with first a gold strike and then platinum. I believe the plating is not the source of the problem.
I would like to try electropolishing to remove the burrs and scratches and sharp points from the wires cut end. What I need are the current/voltage settings and the bath solutions make up. I hope that this is an easy enough process to add because this is something that will have to be done in house. I have a voltage and current controllable power supply and can make up most solutions here.
Any help with this would be greatly appreciated.
Larry JanseniSense co. - Portland, Oregon
2001
2001
A. Monel can be electropolished with phosphoric-sulfuric-hydrochloric type bath, US Patent 2,440,715, Battelle Development Corporation.
Nichrome can be electropolished in methyl alcohol-nitric as given in Durney's Electroplating Engineering Handbook ⇦ this on eBay, AbeBooks, or Amazon [affil link] . A literature review may turn up more.

Tom Pullizzi
Falls Township, Pennsylvania
Q. I'm apprentice engineer in materials and I must perform electropolishing on stent made with Nitinol. If someone could help me or give me some advice concerning cathode, electrolyte and conditions of the experiments (current density, temperatures or else).
Thank you,
Stephanie Garbez- France
2002
A. I do not know what stent is, but Nitinol is a titanium-nickel alloy and is a shape memory metal. I think the only way to electropolish this type of metal is to use a perchloric acid based system, but be warned this can be highly explosive, so before you do it, make sure you know EXACTLY what you are doing. A simple solution would be 750-900 mls/l acetic acid ⇦ on eBay or Amazon [affil link] and 100-250 mls/l perchloric acid, operated at 20-100 V for between 1 and 5 minutes. There is an alternative process called tampon electropolishing; this can be done on titanium alloys using a mixture of 11 mls perchloric acid with 66 mls methanol and 24 mls butyl cellosolve. A voltage of 26-28 V is needed. I would strongly recommend you ask a chemical supply house before embarking on this project.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
2002
A. Hi. I would re-emphasize Trevor's warning against perchloric acid. The worst finishing shop disaster in American history -- with 17 people killed, 150 people injured, and over 100 houses suffering damage -- was the explosion of an electropolishing shop which used perchloric acid. Please see thread 9408.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I want to polish small pieces on Nitinol (50% nickel, 50% titanium). Any suggestions on an electropolishing solution to use?
Thanks,
John Miller- Wilmington, Massachusetts
2002
A. Nitinol can be electropolished in strong acid solutions, (but we do not deal with hazardous technologies) or it can be electropolished in salty bath but with special equipment. Surface needs to be prepared mechanically for about 1 micron roughness. After that we electropolish to mirror reflection. You can send us nitinol parts samples polishing.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
2002
Black substance on wire (electropolished Nitinol)
2003Q. I am electropolishing Nitinol wire and have noticed a black substance adhering to the wire after changing the etchant solution. Suspecting a contaminate in the solution I emptied and mixed another batch. SAME THING! An operator says he remembers a similar occurrence some time ago but this is the first time I've noticed it (recent hire).
The stuff adheres to the wire in globules, is sticky, varies in viscosity and is removed with very hot water. What is this stuff and where is it coming from?
Also, help on what parameters to monitor polishing solution and what equipment to perform these tasks would be helpful. This process is a hard one to manage and I love to be able to get some control out of it.
Kevin Hammedical devices - Sunnyvale, California
? Kevin,
Can you fill in some blanks? Does the Nitinol have a dark oxide on it? Are you removing it before electropolish? With what? What is you electropolish solution and other operating parameters? Electropolish of Nitinol is a dark art.
Jon Quirt- Minneapolis, Minnesota
2003
A. It is not so hard to electropolish Nitinol wire to a surgically clean surface if you use special high-voltage equipment. It takes a few minutes, and the solution is environmentally safe and long-lasting.
As to Nitinol stents - it is a little bit more complicated since it requires to remove laser-made melts from the inner surface.
We experiment now with two-steps process:
first conventional electropolishing (but also in environmentally safe electrolyte), an then final touch by high-voltage to obtain completely clean surface.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
2003
Q. Hello,
I'm apprentice engineer in materials and I have to perform electropolishing on very small and thin parts made of nitinol. I tried so many times to use the 20% H2SO4 & 80% methanol
[affil link] but it doesn't go well.
I heard that electropolishing can be done in acid-free solution, low voltage. Can someone help me or give me some advices concerning cathode, electrolyte and conditions of the experiments (current density, temperatures or else).
Thank you so much,
student - Haifa, Israel
2006
Q. We have been electro-polishing nitinol components and have been observing surface issues. The source of the parts have very small depressions that are smooth. It almost appears like those spots were more polished than the rest of the surface. We remove all oxide from the parts before polishing using an aluminum oxide micro blasting process. Could there be some interaction with the aluminum oxide with the polishing electrolyte solution?
Any help would be appreciated.
Thanks!
Medical device mfgr - Woburn, Massachusetts
2006
A. We do not think that these spots are caused by interaction of Aluminum Oxide with the surface. It looks like pitting, which is caused by changes in electrolyte composition or electropolishing regimes, or by wrong oxide removal procedure. If electrolyte contains methanol, composition of such electrolyte should be very strictly maintained.
adv.
In our methanol-free, acid-free electrolyte for electropolishing nitinol stents we never have pitting problems, and we do not need strict electrolyte composition and polishing regimes maintenance. Contact us if interested.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
2006
Electropolishing of NiTiAu
2007
DANGER!
Please see
previous
warning
earlier on
this page
about use of
Perchloric
Acid!
The internet is a giant one-room schoolhouse where people may read or overhear details about operations that they are NOT equipped or qualified to attempt. Specifics you read here may be intended for qualified chemists with haz-mat training, and working in fully equipped labs.
Q. Dear all,
I am currently looking for a suitable electropolishing solution for the ternary shape-memory alloys TiNiAu in order to have a thinned sample for electron microscopy.
I already tried several solutions that are known to work well with TiNi. Unfortunately, the added gold at a concentration around 15 at% seems to severely hamper the electro-polishing: the same conditions as for Ni-Ti give etched specimens with bad edges of the hole.
We've played with tension, temperature, fluid speed,etc.. but without success.
Solutions tried that work with NiTi but not with my sample:
80% methanol + 20% sulfuric acid (-17°C)
93% acetic acid + 7% perchloric acid (5°C)
93% methanol + 7% perchloric acid (-10°C)
75% methanol + 25% nitric acid (-22°C)
I already looked up in the literature and couldn't find anything close from my alloy. So I was wondering if you might have a suggestion of an alternative, eg, adding an extra acid to one of the above baths or maybe something completely different.
Many thanks in advance,
Rémi Delville
EMAT - Electron Microscopy for Material Science - University of Antwerp, Belgium
PhD Student - Antwerp, Belgium
December 7, 2009
A. I am polishing (Electropolishing) NiTi with high surface finish and have been doing for last three years.
Use the following recipe
90% metahnol and 10% perchloric acid solution.
Temp of polishing 0 °C.
18 Volts for 20 secs.
Before Electropolishing, manual polishing followed by diamond polishing
Try it and let me know if it works
- IIT Bombay , Mumbai, INDIA
Spec for Electropolishing Nitinol?
Q. Is there an ASTM standard for electropolishing titanium alloys? (Nitinol) to be specific?
Dan RueQA Manager - Brooklyn Park, Minnesota, USA
July 14, 2010
Q. I am getting up to speed on electropolishing and wondering if anyone can provide (or point me toward) the specific chemical equations for what is actually happening (dissolution of metal at anode, oxide layer formation, side reactions, etc.).
Benjamin SeimEngineer - Minneapolis, Minnesota, USA
April 27, 2011
How to clean and electropolish wire-knitted nitinol stents
Q. I came across some discussion on Web that nitinol stents made from knitted wire, cannot be cleaned and electropolished. Is this correct? Any suggestions?
Will Servproduct designer - Duram, North Carolina
January 20, 2011
? What reasons were given. While it may be difficult, I think that it can be done. The electropolish may take a lab quality setup to do it. There may be medical reasons why it is not done.
James Watts- Navarre, Florida
January 20, 2011
Q. The person that does outsourcing for nitinol stents electropolishing claims that it is difficult to electropolish areas between tightly knitted areas. There always be spots not polished where wire touches another wire.
Will Serv [returning]product designer - Duram, North Carolina
January 21, 2011
January 26, 2011
A. The wire stents can be perfectly electropolished by Ultra-Polishing method. Even the surface of contacts is shiny without holder-marks. This is an effect of Ultra-Polishing, which differs from regular electropolishing.
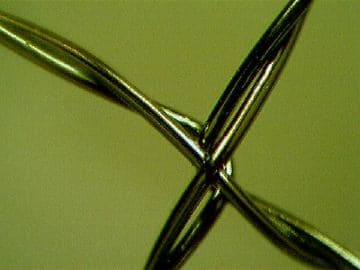

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
Process of ultra polishing
July 15, 2015Q. I would like to have details of the process of 'Ultra polishing' of surgical instruments of SS grade 410/420. How do I undertake this process? Would also appreciate response from people carrying out this process in Mumbai. Of late some of our customers have been complaining of rust in instruments like scissors, artery forceps & some other types of forceps.
Varsha Shetty- Navi Mumbai
A. Varsha,
There are different ways to make ss alloy 410 or ss alloy 420 corrosion resistant after numerous sterilization processes.
adv. One way is to electropolish in Universal electrolyte. Our tests indicate that electropolished by UA surface of stainless alloy 400-series withstand even impact of diluted hydrochloric acid for 10 minutes (normally 400-ss does not stand impact of diluted HCl) . We have licensed our UE to various medical companies all over the world. Sorry we do not have any division in Mumbai.
Another way is to plasma electropolish instruments. Plasma can be of high voltage and of low voltage original This is more expensive method for mass production. We use it only for high-yield products or for parts that require sharpening while electropolishing.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
July 16, 2015
Q. Give me the methods of electropolishing of nitinol stent and heart valve to make surface area clean and smooth. And give the proper parameters of EP. We have the parameters but from that we have not getting proper result so please kindly provide me best parameter.
dheeraj mishra- vapi,gujrata,indai
March 16, 2017
Metal to contact NiTi-anode during electropolishing
Q. Hello everybody,
I'm doing my student research project on electropolishing small NiTi structures. I have several electrolytes like sulfuric acid + methanol. As cathode I use stainless steel.
Can someone recommend a metal to me, which is suitable to contact the NiTi workpiece? (The metal is going to be in touch with the electrolyte as well)
Kind regards
- Germany, Stuttgart
October 26, 2017
October 2017
A. Hi Matthias. Bare copper rack tips are often used in installations for stainless steel electropolishing, and I'm not immediately seeing any particular reason they cannot be used for electropolishing NiTi in a sulfuric + methanol solution. Except for the exposed racking tips, the rest of the device should probably be made of copper but plastisol coated. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi I'm currently working on an electropolishing station for a nitinol implant. I'm using an ethylene glycol solution with a 2% concentration of sulfuric acid and 1 mol of salt, all of the substances reagent grade.
Right now I'm using a stainless steel mesh and platinum coated tweezers as anode and cathode -- are those materials okay?
I'm running at a temp of 20 °C; should I go colder?
For power settings I'm using an amp control with adaptive voltage, should it be the other way around?
Thanks in advance!
Juan
- Salt Lake City, Utah, USA
June 4, 2019
A. Hi Juan, I missed this post back in June.
Nitinol (NiTi) stents electropolishing is not as easy as electropolishing stainless steel stents. If you go with traditional methanol-containing solutions, you have either to invest in special expensive equipment that maintains strict temperature and concentration control, plus prevents harm to you, especially to eyes or
adv.
you can buy the safe process from the company who licenses and teaches how to use their proprietary technologies to the medical cutting shops.
Contact us directly.

Anna Berkovich
Russamer Lab - Pittsburgh, Pennsylvania
(ed. update July, 2025: sorry, Anna has retired)
November 18, 2019
Racking for Electropolishing Nitinol Medical Devices
Q. Good day everyone! I'm manufacturing engineer at a small company for implantable medical devices. We're improving our electropolishing process as best as we can, but I find that we're getting "clip marks" from using reverse action tweezers on our metals (Nitinol mostly). Does anyone have advice for a better fixturing method / clipping device that doesn't give us this under-polished surface where the tweezers lie.
Nicole Jones- New Jersey, USA
November 22, 2019
A. Nicole
If you are using a proprietary EP solution, check with the manufacturer for a recommended material.
If a home-brew, consider copper or copper based alloys.
Maybe post a picture or sketch of the contact area(s) for possible design recommendations.
- Green Mountain Falls, Colorado
December 4, 2019
Q, A, or Comment on THIS thread -or- Start a NEW Thread