-----
Cleaning jewelry with the stones present
Q. I need to be able to clean normal gold and silver jewelry with the stones (if present), as well as the occasional base metal piece. Electrocleaning works much better than anything else to date in removing old cold cream, hand lotion, etc.
The major problems are related to pH. Where some base metals are present, they either dissolve or turn black. The second problem is solvable with rubber gloves, but handling small pieces becomes a serious problem.
Do you know of any formulations that are closer to neutral than the usual sodium carbonate or hydroxide mixes aside from buffering? If not can you suggest places in which to start looking ?
Thank for your time.
A. Hi, Lloyd. I don't have the answer to the perfect chemical to use, but do have a couple of warnings for readers: first, remember that pearls are not stones and may be subject to attack by chemicals. Second, don't mix metals in these solutions as gold may deposit on silver, silver on copper, etc.
While handling small parts might be difficult with chemical gloves or even dishwasher gloves, thin latex gloves
⇦ on
eBay or
Amazon [affil link]
shouldn't be a serious issue -- dentists routinely perform root canals while wearing them.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
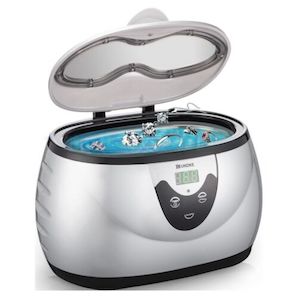
A. For cleaning jewelry, use an ultrasonic jewelry cleaner
⇦ on
eBay
or
Amazon [affil link] with a mild alkaline solution -- Alconox ⇨
is easy to get -- or something that has a little ammonia
⇦ on
eBay or
Amazon [affil link] , there's one called Micro ⇦ on
eBay or
Amazon [affil link]
. Even Dawn dishwashing detergent might do the trick. The key is to give it some time for the cleaner and ultrasonics to break through the built up oils, makeup, etc. Normally five minutes is a decent cycle. Rinse well when the cleaning is done.

Bill Vins
microwave & cable assemblies - Mesa (what a place-a), Arizona
1996
Q. Hello,
I would like to know what household products (if any) would clean silver jewelry the best as well as gold?
2001
A. To answer your question regarding any household products to clean silver.
I saw this on an old episode of Martha Stewart.
Boiling water
Baking soda [in bulk on
eBay
or
Amazon [affil link]
Aluminum Foil
Glass pan
Take your glass pan and line the inside with tin foil.
Pour enough baking soda to line the whole bottom of pan.
Take your silver and put it in the baking soda.
Bring your water to a boil, and pour over jewelry.
Immediately you will see the chemical reaction cleaning the silver. Let it sit in the boiling water until it stops bubbling!
Hurray Clean Silver!
- Santa Monica, California
2005
Ed. note -- Readers: Please see also topic 4785 which includes dozens of comments and details on this process.
![]() That Martha Stewart trick with the glass pan, tin foil and baking soda. That worked awesome. I thought my silver jewelry was destroyed. With this trick and a toothbrush my jewelry looks brand new. Thanks for the great tip.
That Martha Stewart trick with the glass pan, tin foil and baking soda. That worked awesome. I thought my silver jewelry was destroyed. With this trick and a toothbrush my jewelry looks brand new. Thanks for the great tip.
- Thornton, Colorado
2006
Ed. note: It's a great procedure, and we're glad Martha Stewart covered it -- but she can't get the full credit: I have a commercial aluminum electrolytic cleaning plate that was manufactured & sold before she was born -- probably before aluminum even became a commodity :-)
Q. I have a 2 piece white gold ring. It's as if it's one ring over another, with very little space in between them, almost nothing can reach in there. The other day I got really bored, and tried to get a piece of thin paper to go in there, and after about 10 minutes I successfully got the corner of it in there. But it came out black :|. What kind of solution can I leave it in, that would not damage the ring but would eventually clean out that stuff in the middle?
John piolawskibuyer - reseda, california
April 10, 2009
A. Hi John,
The idea behind commercial jewelry cleaning machines is that ultrasonic energy introduces cavitation (miniature reverse bubbles, vacuum bubbles) which pull cleaning solution in, then pull dirt and cleaning solution out.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread