
-----
Chemical Drop/Black Spot on Alkaline Zinc Plated Part
Q. A chemical drop problem occurred on a piece plated with alkaline Zn and chromate (III) yellow. The hypothesis is that this likely occurred during the process before the piece was plated. Based on SEM-EDS results comparing the chemical drop point to a normal surface, the drop point showed low Cr and high Zn, while O and C tended to decrease compared to the normal surface. Therefore, it is suspected that an alkaline substance had adhered to the piece, reducing the Chromium(III) adhesion. A pH paper test on the chemical drop point revealed a level 12 alkalinity.
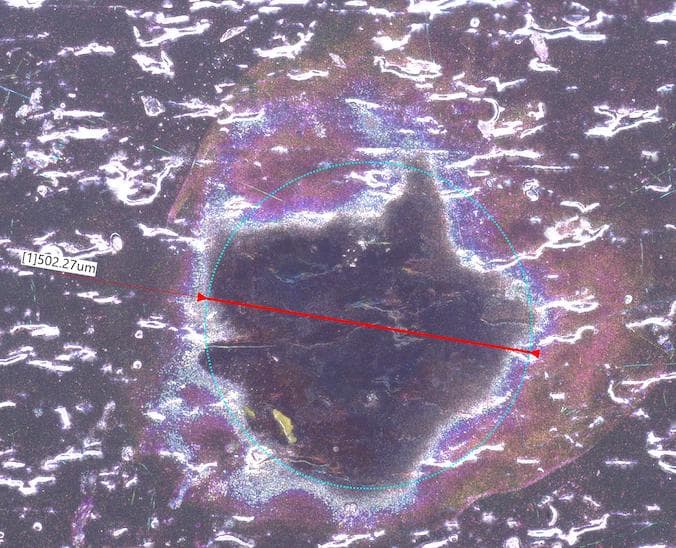
- How should I check to see what solution or chemical the chemical drop is from?
- I don't know what caused this chemical drop, whether it was a drop, a mist, or something else. What should I do to find out?
- I have simulated a chemical drop in the lab by dropping a solution from the plating bath or by simulating bubbles and then placing a test strip against the bubbles. When the bubbles burst, a black defect appeared, similar to a real chemical drop. However, it did not appear as a round spot and the same size as the actual Chemical drop.
Students - Thai
September 8, 2025
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
A. You have made a reasonable conclusion that it was a drop of something that is alkaline. Why would you need to know specifically what chemical caused the defect? A caustic etch would be strong enough, but it could be something else.

Jon Barrows, MSF, EHSSC
Kansas City
September 9, 2025
Q, A, or Comment on THIS thread -or- Start a NEW Thread