
-----
How to achieve Brown Zinc Chromate finish?
Q. Hello Again. Here is another item looking for help. How to achieve or purchase dichromate crystals of a brown color, not a reddish brown more like a root beer color. If need be, who does this.
This color is also a highly desirable finish used on Chrysler muscle car parts form 65 to 73. No one can seem to produce this finish.
- Pompton Plains, New Jersey
August 14, 2012
A. Hi, Bill. Readers might not understand a verbal description. Please try to send pics if you can. Thanks!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
August 17, 2012
November 10, 2014
Q. Attached are some pictures of 2 different Chrysler original new never used or subjected to sun or bright lighting.



Base is definitely zinc or cad. Probably zinc. On the backside of the bigger catch you will see a spot where I applied few drops of 50% Muriatic acid & water. Took approx. 10-15 seconds to get to bare metal. The brown chromate does not scratch easily, as blacks do.
Can anyone shed any light on this question?
Bill Meerholz [returning]- Pompton Plains, New Jersey
What is brown zinc that was used on Chrysler parts late 60's-early 70's.
Q. What is brown zinc that was used on Chrysler parts late 60's early 70's. Hood latches, etc.
Bill Meerholz [returning]Restore many original Chrysler parts including hood latches that are the brown zinc finish. - Pompton Plains, New Jersey
October 23, 2016
Q. Can someone please advise something about this what is believed to be brown chromate or what it is or how to achieve it? What is the finish on latches pictured?
Bill Meerholz [returning]- Pompton Plains, New Jersey
January 8, 2017
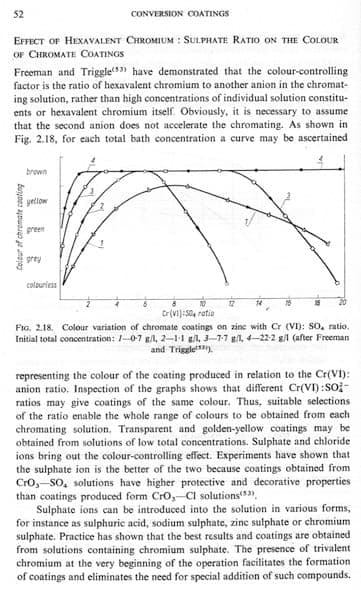
by Biestek & Weber

on Amazon (rarely)
or AbeBooks (rarely)
(affil link)
A. Hi Bill. Nobody has related their experience with this finish in 4-1/2 years now. There are two ways to get varied colors in chromating: dyeing the chromate or changing the operating conditions. The attached page from Biestek & Weber.
implies that brown chromating can be accomplished, without dyeing, by adjusting the sulphate ratio. I'm not sure how much practical help that will provide though :-(
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
January 2017
The dark chromate conversion coating described and seen in the photos is one of two possibilities: (1) an olive drab chromate, or (2) a dark yellow chromate. In the more common yellow (or gold) chromates, if an article is (a) left in the chromate for a longer period of time, or (b) the chromate is too concentrated, or (c) the pH is low, then the chromate will be dark, running towards brown. If the chromate is too dark, the chromate conversion coating will be friable and powdery, so these processing conditions are wisely avoided.

Tom Rochester
CTO - Jackson, Michigan, USA
Plating Systems & Technologies, Inc.

January 24, 2017
Trying to identify a 1950s anti-corrosion automotive metal finish
Q. Hi
I own a 1956 VW Type 2 that, having lived most of its life in a dry climate, is mostly rust free and retains its original anti-corrosion coating in areas that were not directly exposed to abrasion and road spray. I'm trying to identify what coating that might be. My ultimate hope is that whatever coating was used, I might be able to reproduce it on repair patches.
The coating is a bronze colour and has a slight satin sheen. It does not appear to be a paint-type coating. It is very thin, does not flake and scratching it reveals bright steel with no primer coat.

Other owners of VW Type 2s of that era have reported finding the coating on the inside of box sections etc, suggesting that the coating was applied by dipping and I have seen period production line photos showing VW dipping Type 2s. A period VW technical document mentions 'phosphatierung' (phosphating), but the coating is not grey and I believe that phosphate coatings are intended to be temporary protection and would have needed additional protection to have survived this long?
Can anyone suggest what the finish might be and whether it could be (safely) applied to modern steel repair patches by spraying or another means?
- Horsham, West Sussex, UK
May 17, 2017
A. Hi Andrew. I'm not quite sure what this component is. It looks like just a frame stiffener, but hollow such that electrical or hydraulic lines, or hot air, could run through it? If so, I don't know why it's stepped or telescoping though. If it's just part of the welded frame, it probably has the same coating the rest of the frame once had.
My guess is it's an early electrocoat priming but it's probably not really possible to figure it out just from the look of it. Hopefully someone will wander in who actually knows what VW did in those days.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 2017
A. Maybe that is some sort of combined phosphate and chromate finish. Hope it helps and good luck!
Goran Budija- Zagreb,Croatia
May 31, 2017
Q. Thank you for the responses.
Am I correct to assume that, if the finish is a brown zinc chromate, it cannot be re-applied or applied to patch areas of repair on my vehicle?
On that assumption, I'll look to try to get a reasonable paint match and paint repaired areas to a suitable dividing point, so it's not so obvious.
- Horsham, West Sussex, UK
June 3, 2017
A. Hi again. Chromate only works on the zinc plating (and a few other metals), not on steel. Further it is toxic and carcinogenic, and I don't think that's what it is anyway. So I think that rust converter
⇦ on
eBay
or
Amazon [affil link]
followed by your paint idea may be the best approach. In the meantime, though, I stumbled upon this informative paper which you might want to review .
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
June 2017
Q, A, or Comment on THIS thread -or- Start a NEW Thread
