
-----
Finish for a copper radiator/heatsink. Cleaning copper & solder
Q. My friend and I are in the process of completing sculptured designs for central heating radiators made from copper tubing ... but we have a problem. The overspill of the silver solder we use to fix the copper cap ends to the pipes needs to be removed prior to chrome plating. However using files plus wet/dry carbon paper is time consuming.
I therefore have two questions to ask
1. Does anyone know of a machine with appropriate accessories to clean intricate metal without deeply scoring the soft copper?
2 Does anyone know of a chemical to clean off the blackening caused by the soldering?
Thanks
designer - Enfield, Middx, United Kingdom
2005
? Could you give us a scope for the size of the pieces you are doing?
Marc Banks- Elizabeth City, North Carolina USA
Q. The ends of the radiators we are cleaning range from 15 mm to 22 mm in diameter, of which there can be up to 12 in a single piece. These are affixed to another copper tube being spaced at between 20 mm to 25 mm intervals along its length. Hope this helps.
Craig Humphreys [returning]designer - Enfield, Middx, United Kingdom
A. I think that this is going to be an experimentation thing, do up some pieces from scrap you can test on. Try a NON-DILUTED solution of muriatic acid
⇦ on
eBay or
Amazon [affil link] to see if it will eat the black residue off, afterward passivate the surface with either lots of water or water and just a touch of baking soda [in bulk on
eBay
or
Amazon [affil link]
. The metal will oxidize up again, but at this point I would suggest a bath of 5% citric acid
⇦ on
eBay
or
Amazon [affil link] . Dip and see if it cleans it up.
THIS IS IN CAPS FOR A REASON, IT'S IMPORTANT.
I HAVE NOT TRIED THE ABOVE METHOD BEFORE FOR EITHER THE CLEANING OF METALS OR SIMILAR.
Be careful when using acids, they are corrosive, they give off fumes, and they can severely damage respiratory functions. That said, give my suggestion some thought, ponder if you have similar (but less dangerous) chemicals available that will do the same.
- Elizabeth City, North Carolina USA
Multiple threads merged: please forgive chronology errors and repetition 🙂
Brightening of copper radiator without harming solder
Q. Dear sir,
I am working in the field of metal treatment. I want your guideline for cleaning or brightening of radiator which is copper based without any proprietary formulation. Basically radiator is copper based, during process of soldering and other heat treatment radiator gets corroded or say tarnished strongly sometimes gets green. I have tried nitric and sulfuric based pickling but problem is there that soldering also get affected. so tell me any process which overcome such problems
Thanks.
radiator manufacture - Mumbai
2006
A. I've used d-rust-it before and it works well on copper corrosion and it is non-hazardous too!
Howard Phillips- Corning, New York
Multiple threads merged: please forgive chronology errors and repetition 🙂
High thermal emissivity and thin coating needed
Q. Hello, I'm looking for advice on what the best finishing would be for a large passively cooled copper heatsink/radiator I've constructed, in order to maximise heat transfer to the surrounding air. Here's pictures and a link for what it looks like:





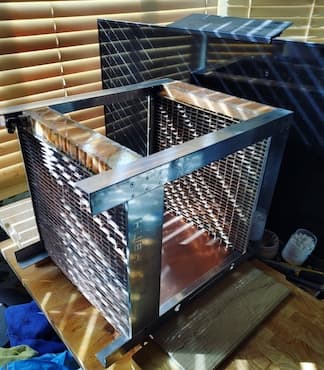
http://forums.overclockers.co.uk/showthread.php?t=18019135
I'm looking for a finish for copper that will has a very high emissivity to increase heat radiation and is a thin coating so as not to decrease conductive heat transfer from the copper to the air too much. In terms of physical properties it needs to ideally not easily flake off when handling the large, heavy heatsink/radiator, but other than that doesn't need to be particularly hard-wearing. Ideally the finish will also colour the tin solder the same or a close colour.
Is there is a tried-and-trusted finish used for passive-airflow copper heatsinks/radiators?
So far I have come across either cupric oxide (Ebonol C) or blackening with liver of sulfur
⇦ on
eBay or
Amazon [affil link]
as possible treatments, but I can't find any information on the emissivity or likely thickness of these coatings in order to judge which would be better, or if there is a better alternative. Any help would be much appreciated!
Hobbyist - Margate, Kent, England
October 5, 2010
Q. Surely someone must have something to input? That's a bit disappointing.
Dr Tom Gravgaard [returning]- Margate, Kent, United Kingdom
October 27, 2010
A. Hi, Tom.
Ebonol C is proprietary process chemistry offered by Enthone, Tom, and I'm very confident that they will have all the numbers you are looking for. More generic chemistry, like liver of sulfur, is not likely to offer the consistency & robustness of a proprietary like Ebonol C, which has been a successful commercial offering for 50 years or so.
Do I correctly understand that your project is about providing fan-less, pump-less cooling (water cooling?) to a high performance computer? Although the CPU gets hot, does this water cooling system really get hot enough that emissivity will play any significant role in the cooling compared to convection? The pictures are taken outdoors -- does the computer or its cooling system actually stay outdoors or does all the heat have to be absorbed into a room? I see a vertical aluminum or stainless steel mounting plate in your second picture -- what kind of stuff get mounted on this? Does the whole computer go here, and the cooling system is the outside frame?
It's unlikely that you could successfully electroplate this as an assembly. Electroless nickel plating might work but would be terribly expensive. You might be able to immersion tin plate it. I doubt that hot tin dipping would achieve much. I think you are on the right track with the Ebonol C :-)
Regards and best of luck,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread