
-----
Crystals developing on solder/copper areas
March 25, 2010
We recently have observed tiny crystals developing on our soldered buss wires that have varying color from green to dark gray. Crystals also appear on the edge of a copper tube, which appear to be bluish in color. This change seems to be coincident with instituting temperature cycling of our PCA's, or this is just accelerating a pre-existing condition.
Our current assumption is that we must have chlorine or sulfur outgassing from "something", but distinguishing whether we have copper sulfide or copper chloride
crystals has not been determined yet.
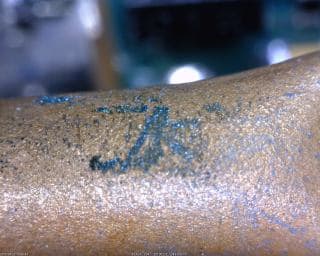




Looking for any inputs of possible sources, i.e. sulfur outgassing from surrounding stainless steel parts, PCA outgassing from supplier process, contaminants in filtered water, etc. and any quick and easy tests that could be done to determine whether we are dealing with chlorine or sulfur (or maybe something else).
Sandra CrelleyMfg Engineer - Redmond, Washington
It's not the right color to be either chloride, which is more green'ish) or sulfide (which is nearly black). It could be a hydrated sulphate or some other anionic salt of copper. I don't have any guesses as to the source, sorry.

Jon Barrows, MSF, EHSSC
Kansas City
March 31, 2010
Q, A, or Comment on THIS thread -or- Start a NEW Thread