
-----
Zinc plating with clear chromate discoloration issues
March 26, 2022
Q. Good evening. Lately we have been having some trouble with a little purple hue after we applied chromate on Zinc plated parts, is there a way to control it? Thanks beforehand!




Cesar Rivera
- Chihuahua, Mexico
(to help readers better understand the Q&A's)
Steel components are often zinc plated for corrosion resistance and sacrificial (cathodic) protection. Because zinc is a more active metal than steel, it will preferentially corrode; that is, when corrosive forces are at work, "stealing" electrons from metals and causing them to corrode into solution as positively charged ions, zinc will donate its electrons to the steel, sacrificing itself to protect the steel.
To deter the zinc from too rapidly corroding away itself, zinc plating is almost universally chromate conversion coated -- a process which passivates the zinc and slows the corrosion. Historically these chromates were usually based on hexavalent chromium, which is inherently yellow, because hexavalent chromium did the job better than anything else. People would sometimes try to leach the color away through immersion in hot water before the chromate cured; but others have felt if there is no color there is no hexavalent chromium, so the approach wasn't valuable. And proprietary formulations of clear or blue chromate did not come even close to matching the performance of yellow chromates.
Hexavalent chromates are toxic & carcinogenic, and efforts to reduce their use have been perennial, but in recent years the European Union issued RoHS (reduction of hazardous substances) standards that effectively prohibited most use of hexavalent chromates in Europe, and all car makers around the world began adopting those standards for everywhere. At the same time, a new generation of high technology trivalent chromates (hexavalent-free) became available that can match the corrosion resistance of hexavalent chromates. Most of these are inherently colorless but may be dyed, so today there is no categorical difference in the corrosion resistance of chromates based on their color, and most chromate is trivalent.
So some of the ideas in this 2007 thread are not very applicable anymore: it is possible to specify clear or blue trivalent chromates that are just as corrosion resistant as the yellow chromates.
A. Hi Cesar.
Daniel_Montañés suggests below that the purple color is due to the chromate being too thin, and I think he's right.
It seems most pronounced in the recessed area, and it's possible I suppose, that either you have insufficient agitation in the chromate tank to handle that recessed or that the plating is too thin in the recess to produce a good chromate. Because the plating surely is thinner in that lower current density area, that would be first guess.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 2022
A. Hi brother Cesar,
As Ted mentioned, I think it has something to do with chromate layer thickness.
You can make thickness more even with agitation and better pH control, and maintaining concentration in your operation range.
We in our plant use the same chromate (trivalent high layer) for blue and iridescent plating. Blue with low concentration and high pH operation range (low thickness, blue hue) and iridescent with high concentration and low pH operation range (high thickness, green/red hue). This can be controlled and results are good if you have the right chemicals!
Best wishes,
Daniel
Process Engineering Manager - Cañuelas, Buenos Aires, Argentina
May 4, 2022
⇩ Related postings, oldest first ⇩
Q. I am Quality Technician of a machine shop in Austin, Tex. We machine components parts for the semiconductor industry. We are currently having an issue with our customer in China with our parts being rejected for a yellowish appearance on arrival of the parts. I would like to know if this discoloration is caused during shipment overseas or is there a flaw in our supplier special process.
Zinc plate with clear chromate per ASTM B633, Type 3, SC 2
Sheet is CRS 1010-1020, .060 thick
Quality Technician - Austin, Texas , USA
2007
A. Lots of problems there, Tommy :-)
1). The people commenting on the appearance are thousands of miles away from each other; although everyone hates 'sample boards' for good reason, appearance standards can't be enforced without them; 2). Politics may be involved regarding whether this rejection is an attempt to erect an artificial trade barrier; 3). The people whose opinions you are enlisting here can't see the parts and don't know if it's rusting, the chromate too thick and streaky, or yellow chromate applied accidentally.
If you can attach really good photos maybe we can at least fully understand the complaint and narrow it down. Thanks, and good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2007
A. Ted is perfectly right, it's not possible to diagnose such troubles with any certainty without seeing the parts in question.
I will say that when I have seen this problem, it has generally been a result of drag in contamination of the bright dip, because of chronically inadequate rinsing after the plating bath. Cutting corners, in other words.
I will add my completely personal opinion in the form of that old bromide...YOU GET WHAT YOU PAY FOR.
Good luck.

Dave Wichern
Consultant - The Bronx, New York
2007
Q. Here are the attached photo's you requested. I just wanted to know if the discoloration appearance of these parts could change during shipment overseas. We keep having this issue even after all parts are 100% inspected.


Quality Technician - Austin, Texas , USA
2007
A. It is either a bad picture or that is poor (bad) plating. I would not call it a clear chromate. You might want to go to a very light blue chromate to avoid the yellow. Your chromate vendor can help you with both the yellow and possible solutions / alternatives.
James Watts- Navarre, Florida
2007
A. I suspect the chromate pH is high due to zinc solution drag-in. In buckets set up a Nitric Acid 1/4%/vol. for neutralization followed by a fresh made chromate. Then cheat a 1/4%/vol. Soluble Oil in the hot water rinse. Then consider that everything that goes thru the Panama Canal does temperature and humidity cycle, so do not wrap in sulfur ⇦ on eBay or Amazon [affil link] bearing brown paper.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

2007
A. One way that some shops attempt to do a "clear chromate" is via a quick dip into a yellow chromate tank followed by leaching the color out in a final warm water rinse. This can easily lead to the issue I see in the photos. Your vendor should have a legitimate clear chromate tank and the first thing I'd probably do is ask him if he does.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2007
A. I agree with all the previous comments although, of course, I would like to add some of my own.
The yellowish discoloration could be due to the parts being processed in an alkaline non cyanide zinc solution that uses older (non polymer) brightener technology. This would be typical. The "fix" is a 0.5% nitric acid pre-dip before the chromate, use of a "very blue" trivalent chromate and close control of the chromate pH.
With trivalent blue bright chromates, increases in the pH that lead to values greater than 2.3 (1.8 to 2.0 being optimal for most processes) results in a thicker, more protective film but the refraction of light off this film will result in a yellowish cast to the part.
The simplest explanation could be just plain old poor rinsing so the part was stained.
The first thing you need to do is talk to the plater and have good communication with them to help solve this problem. The plater may chose to ask his vendor(s) for advice. Now you have knowledgable (I hope) people working to solve your issue. Of course none of this does any good if you also have a communication problem with your customer!
process supplier - Great Neck, New York
2007
December 25, 2014
A. Hi Tommy,
Your blue/clear chromate parts yellow at the edge , I think your nitric acid dip tank is contaminated; make a new 0.5% by vol. nitric acid dip and try again.

Popatbhai B. Patel
electroplating consultant - Roseville, Michigan
Q. Is there a difference between Clear vs. Clear, blue bright?
We are trying to come up with a standard finish note that is ROHS compliant.
FINISH: CLEAR, CHROMATE PER MIL-DTL-5541
[⇦ this spec on DLA]F, TYPE II - CLASS 3.
or
FINISH: CLEAR, BLUE-BRIGHT, TRIVALENT CHROMATE PER MIL-DTL-5541F, TYPE II - CLASS 3.
Would this be saying the same thing or would this be 2 separate notes, stating different colors?
Designer - Wilmington, Massachusetts
March 12, 2008
A. You are the one who must decide what you want, Richard, so you must see to it that if someone follows your spec you will get what you want. So the question then becomes, if parts come in somewhat blue-ish would you consider them satisfactory? If they come in with no coloration at all would you consider them satisfactory? If yes, then either spec can be used, no worries.
If you have an appearance standard in mind, you can make sample boards showing what is acceptable and what isn't. People hate sample boards for good reason though -- one of which is that nobody wants to go through the effort of re-mobilizing and re-doing the whole effort, so the sample boards often don't get replaced as they deteriorate, and soon people are comparing the current parts to their recollection of what the piece on the sample board "used to look like" before dust & oxidation & corrosion set in :-)
Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 13, 2008
Leaching conversion coatings on Zinc plating
Q. I do a small amount of zinc electroplating at it applies to auto restoration in a hobby-only setting. Is there a simple clear chromate conversion formula for treating the parts after zinc plating? I plan to use a sodium di-chromate/ sulfuric acid formula as a conversion coating, but I need a clear finish on some parts, not yellow/gold. Will a leaching dip of some sort remove the yellow/gold/iridescent color, yet leave the protective conversion coating? I came across a note somewhere that vinegar
⇦in bulk on
eBay
or
Amazon [affil link] could be used to remove the coloration but retain the protection of the conversion coating, is this true?
Always fun coming here, reading through posts by professionals, trying to understand what the heck you guys are talking about :)
Thanks,
JohnnyB
Hobbyist - Saratoga Springs, New York, USA
January 15, 2014
A. Hi John. Last item first: we've started putting a "QUICKSTART" sidebar on some our pages to try to address the problem of the threads being hard to follow. With 50,000 threads it will take forever, but at least it's underway.
Leaching will reduce the yellow saturation and the amount of hexavalent chromate and the corrosion resistance. vinegar is bad stuff to put on zinc, as it is a mild acid and zinc plating is not at all acid resistant. Professionals use only proprietary trivalent chromates, and I'd suggest you try to do the same. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
January 17, 2014
Clear chromated parts are yellowish, especially at the edges
Q. Hi there. I work at a plating shop as well and I have a similar problem: I process the steel pieces and when I'm ready to clear chromate them I tried several different immersion times, and I get yellow all over especially on the edges. What could it be if temperature is good and pH is good ?
Luis Melendez- haverhill, massachussetts
December 9, 2014
June 5, 2016
A. Sorry, but many of the answers above are not convincing enough about yellowing of clear chromate Zinc parts.
A number of things can happen.
1.- Discoloration is a result of over chromating times 10-15 secs., depending on pH.
2.- Tank too deep so chromating solution too long in contact with parts.
3.- Blue Chromate too low pH.
4.- Drag out solution over-concentrated, or Rinse tank too high pH.
5.- Poor Zinc plating coating
Answer. Best way of tackling this problem is by testing all areas of the process separately and step by step. Small set of containers (5 liters)each will allow you to test all your processes with clean rinsing water, new chromates, timed dipping times and so on & so forth. If you are an electroplater, that is easy and effective, so you can produce regular top quality product finish consistently. Otherwise refer to your chemist for assistance. Hull Cell ⇦ huh? can also help.
Jenaro MincholaElectroplating - Melbourne, Australia
Purple Hue on Clear Chromated Parts
Q. I work at a plating company and we use Dipsol 444 products and we are seeing purple hue.
Do you know what causes this and how to fix it.
Lori
Quality Personal - Barney North Dakota USA
July 17, 2017
A. Hi Lori,
Purple hue is indicating you a low thickness chromate conversion layer. Colors range from red (the thinnest) to green-yellow (the thickest). Blue is in the middle, and that iridescence is very common in trivalent conversion coatings.
You can modify that hue modifying your operational parameters in your trivalent conversion coating bath, or applying some sealer. If I may, I would ask your vendor how to manage a clean bright color without hue or with a blue hue, if you don't need high corrosion resistance.
Hope this helps you! Regards,
TEL - N FERRARIS - Cañuelas, Buenos Aires, Argentina
August 7, 2017
Q. Hi all. I am Durkesh and, I am working in a Sheetmetal fabrication industry and we are doing Zinc Plating with clear chromate as per ASTM B633 Type III, SC2, and some of my parts are yellowish after chromate. We run two different parts (base material Electro Galvanized Steel and CRS) at the same time but the outcome is a different colour (EG is good Blue colour but CRS becomes yellowish). Both are plated & chromated in the same tank and same timing. Can anyone please advice me to get consistent colour every time?
Process : Alkaline Zinc Plating
Chromate pH : 2.2 - 2.3 (maintained as per supplier advice)
Chromate Timing : 18 Sec
- Selangor, Malaysia
June 11, 2020
June 13, 2020
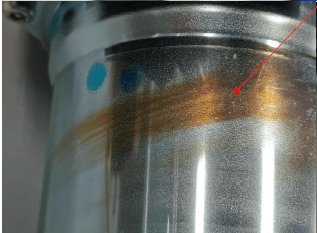
Q. Sir,
We are encountering problem in our zinc plating blue trivalent conversion coating. The problem is that some of the areas turned brown when it was already assembled and shifted abroad. Do you have any idea on what phenomenon causes such appearance?
Our bath is non-cyanide alkaline zinc type. Thank you.
Kevin Sosa- Philippines
June 16, 2020
A. Kevin,
Your picture shows a cylinder that I can only assume is hollow so there will be some unplated areas of the ID. Not only will those unplated areas start to dissolve in a passivate (pH is typically around 2-2.5), but the zinc coating itself will also start to dissolve. These two metallic contaminants can affect salt spray results/corrosion protection and discolor passivates. The yellow/brown streak can potentially be high iron in the passivate (150 ppm or above). To test that theory you could run sample pieces in your zinc bath and then, offline in a freshly made sample batch, passivate the piece. This sample passivate would not yet be contaminated. Alternatively you can have your chemical supplier analyze your bath for metallic contaminates, typically iron and zinc.
On another note, the mark seems to follow a pattern that would be visible if the "tail" was facing towards the surface of the solution. This can be indicative of poor agitation in the chromate as well.
Ultimately we need more information to help you answer this question. Typically we like to ask questions of the applicator such as:
-Is this happening on all parts or is it only a fraction?
-Is this happening in the same place on parts or is it random?
-How are the parts processed from preplate to postplate and anything in between that may help.
- Cleveland Ohio
June 19, 2020
Q. Sir I Brown,
Thank you for replying to my query.
The appearance happened only on specific dates and not all parts have such brown appearance after shipping abroad. I have checked the contaminant on those dates since we have monitoring for Fe and Zn contamination on our chromate passivation bath. There is only one day in which the ppm is over 100 for Iron contamination (130 ppm to be exact).
The appearance of brown discoloration seems to be located on different part of the item.
The process involves automatic dipping since we are on an industrial set-up for plating.
Preplate: Degreasing -> acid etch -> Iron activation (hydrochloric acid)
Post plate: Activation (nitric acid) - > chromate passivation -> drying
- Philippines
June 19, 2020
A. Kevin,
At 130 ppm I would consider that starting to enter the danger zone for iron contamination. I think 150 ppm is more of an "absolute" upper limit, but at 100-150 you can experience problems. Of course every shop and each individual process is unique so that may not be 100% accurate for you. Also, different passivates have varying levels of complexing agents to accommodate for more or less contamination. I still suggest trying the sample with a fresh make up compared to your "as is" solution to show how no iron can improve appearance.
When you say the appearance is on different parts that is not very descriptive. From your responses I cannot tell if this is barrel or rack (I am guessing rack though). If it is a rack line the more description would include:
-Is it happening on all parts on a single rack?
-If it's only happening on some, is it happening more near the top/bottom/outside/inside of the rack?
Note: I'm not asking arbitrarily -- it can lead the troubleshooting towards different avenues based on the prevalence of the issue.
If you are using an alkaline non-CN bath then your post plate sounds good as long as you have an initial dead rinse tank after the plating tank followed by a flowing rinse and then your nitric predip (0.5% v/v typically) before going into the passivate. If the issue is not being seen on parts as it comes out of the chromate and only after drying then I am even more convinced it is iron related. Again, I stress doing off line testing with fresh passivate to determine this quickly and economically.
- Cleveland Ohio
Q. Hi all I am Durkesh and I am working in a Sheetmetal fabrication industry. We are doing Zinc Plating with clear chromate as per ASTM B633 Type III, SC2, some of my parts are yellowish after chromate ( we run two different ( Base material is Electro Galvanized (EG) Steel and CRS ) parts at a same time but the out come is different colour ( EG is good as Blue colour and CRS is became yellowish ) both are done plating and chromating in a same tank and same timing. Can anyone please advise me to get consistent colour every time.
Process : Alkaline Zinc Plating
chromate pH : 2.2-2.3 (maintained as per supplier advice)
Chromate Timing : 18 Sec
- Selangor, Malaysia
June 11, 2020
Q, A, or Comment on THIS thread -or- Start a NEW Thread
