
-----
Is co-deposition of silver-Germanium alloy possible for silverware?
Q. Has anyone successfully done any research on the co-deposition of silver-germanium alloy and have any commercial applications been successful ?
This maybe the best solution for a long term anti-tarnish property since most of the proprietary anti-tarnish dips have a very limited life; chromate base dips lead to yellowing over time and also lead to effluent treatment issues besides being toxic (at least pschycologically ); Tarniban is chromate based; electrophoretic or other alloys are expensive besides leading to yellowing over time and cannot be applied to food based products; etc. So, if a Silver-germanium alloy can be deposited, it MAY be the best solution.
Hopefully somebody will enlighten me on this alloy coating.

Deepak Whorra
silver craftwork - New Delhi, India
2007
|
In USA and Europe you can buy proprietary silver-copper-germanium alloy (Argentium(R) sterling silver) and according to producer it is very resistant to tarnish. You can buy some of alloy and dissolve it in cyanide solution, then you can try to electroplate with obtained solution, but probably things are much, much more complicated. Hope it helps and good luck! Goran Budija- Cerovski vrh Croatia Deepak, Process Engineer - Syracuse, New York |
Q. Hi, Thanks for your replies... I do have some of that alloy but how do I plate it - is Germanium not a semi-conductor ? How do I dissolve that in the cyanide bath? In any case I will experiment and then inform you guys of the results.
In 1993 it was Marc De Bonte who conducted that experiment. I wanted to know if anything further has been done subsequently because the alloy itself is very tarnish resistant...can it be deposited ?
Thanks for your responses but would look forward to answers to these questions.

Deepak Whorra
silver craftwork - New Delhi, India
2007
by Abner Brenner

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Deepak.
Brenner's "Electrodeposition of Alloys" discusses this a little bit, referencing:
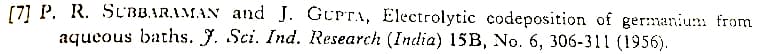
Briefly, it seems like a silver cyanide bath with a little germanium oxide in it can generate a deposit of about 90% silver and 10% germanium.
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread
