
-----
Preventing Red Spots and Reddish Soft Gold Plating
Q. We presently plate mid-P EN (3~5microns) on a kovar base material. This follows with, electrolytic soft gold (cyanide based, 0.6 microns minimum). After the drying process, occasionally red spots appear on the surface of the gold layer. Even rinsing with DI water at 18 megaohms does not seem to help clear away the red spots. I am looking for a way to eliminate this problem.
L.Poobalanprecision finishing - Singapore
2006
A. Have you tried a hot DI water rinse? Cyanide based baths are hard to rinse. Cold water rinses tend to congeal salt residues on the surface. I would make sure the gold salts are fully dissolved in hot DI water before making your adds to the bath. A hot DI rinse will also aid in faster drying as well. If using a spin dryer it's also a good idea to dedicate a dryer strictly for gold plated parts to avoid cross contamination. Good Luck!
Mark Bakerprocess engineer - Malone, New York
Q. Hi guys,
I need some assistance to solve this problem: what are the probable causes of reddish gold after electroplating? This problem is intermittent to all circuits that we plate here.
Process Manager - Cavite, Phillipines
2007
by C W Zanariah Ngah

on eBay or Amazon
or AbeBooks
(affil link)
A. Cesar,
You don't mention what type of gold you are plating. Is it a hard acid gold that is cobalt or nickel hardened? A red deposit can be caused from too high a current density, too low a gold concentration in the bath, improper ph, bath contamination whether it is organic or inorganic.
1) Make sure the Au concentration and current density are both within spec.
2) Check the pH.
3) Double check temperature of the bath.
4) Make sure the brightener level is in range.
5) Have the bath checked for metallic (inorganic) impurities.
6) Put a carbon filter in the filter chamber, run for two hours, repeat process with new filter. Good Luck!
process engineer - Malone, New York
A. Hi Cesar,
Could it be a case of burnt gold plating. Please check the gold content and adjust (increase) as necessary. If you are worried about higher gold consumption due to drag out losses and prefer to operate at slightly lower gold content, you can operate at lower current density with corresponding increase in plating time to achieve the thickness required. Try increasing the solution movement as well.
regards,
- Manila, Philippines
2007
Q. Hi Mark;
I use a soft gold bath or acid base gold.
Regards,
- Cavite, Philippines
by Reid & Goldie
-- hard to find & expensive; if you see a copy cheap, act fast!

on eBay or Amazon
or AbeBooks
(affil link)
A. Cesar,
I would still follow all suggestions instead of #4 (determination of brightener level). Germie Maravella had a good point regarding increasing the solution movement as well.
process engineer - Malone, New York
A. Dear Sir,
It looks like copper contamination in the gold bath. However, please be sure of prescribed pH and Current and Voltage densities.

Shafiuddin A. Mohammed
metal coating shop - Dubai, United Arab Emirates
2007
Q. Dear sir.
We are doing the Electrolytic gold plating DIRECTLY on COPPER for our microwave applications.
Whereas we are not getting the yellow colour of gold,
maybe the reason is some amount of copper is dissolving into the bath?
Pl. let me know how to remove impurities/ contamination/ metallic particles (copper), etc?
- Hyderabad, India
December 16, 2011
A. Hi,
Reasons for reddish tint...
1. Low gild metal content in the bath
2. High pH
3. High Temperature
4. Impurities in the bath.
Hi Mark ..good to see you back..if you are there most of the precious plating sections are taken care....Season's Greetings!

T.K. Mohan
plating process supplier - Mumbai, India
A. Jhonson, before you start trying to remove impurities from you gold bath, do an analysis to find out what impurities you have in there. Also do a Hull Cell ⇦ huh? test to see whether it is the gold solution of the gold thickness at fault. What thickness of gold are you actually depositing?

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Q. Hello,
I am quality engineer and would require your feedback and recommendations on a discoloration issue I am finding in one of our parts.
We are plating Gold over Nickel on a Copper base material part. The Nickel plating is as per spec AMSQQN290 Class 2 and the Gold plating is as per spec ASTM B488 Type III Code A; thickness 0.00001 (10 micro inch).
The discolorations observed on the gold plated area; like brownish (burnt) or purplish color instead of the yellowish color, which we see for gold as part of the visual appearance. This brownish / purplish color will be gone when brushed using a brass wire brush
⇦ on
eBay or
Amazon [affil link]
slightly and is visible on 100% of the parts plated (the qty plated is 300 parts).
Since the gold thickness is low and gold plating is a "Soft Gold", the plating is done in the gold strike bath (no brighteners added in the gold strike bath).
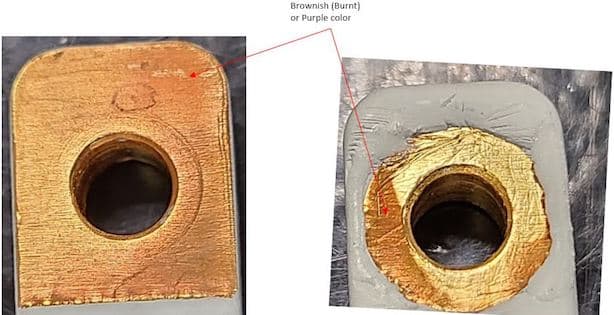
Please provide your feedback on this issue and let me know what would be the recommendations to prevent the purple/brownish (burnt) color, as we have more qty to be plated. Looking for your valuable feedback/recommendations.
Thanks,
- Chicago, Illinois
February 23, 2021
A. Hi Elias. We attached your inquiry to one of several threads we have on discoloration of gold plating, and there should be food for thought while you are awaiting further input. But, unless we are misunderstanding each other about the implications of "strike", I think a big part of the problem is the attempt to use a gold strike bath as a decorative plating bath.
• It's not designed to be decorative and it's not designed for deposits of 10 microinches.
• Once it's been in operation a while it will be high in nickel and other contaminants because it's designed to activate the nickel by dissolving light oxides. Contaminants can sometimes create gold alloys of brown or purple.
• Strikes are designed as 'garbage collection' steps to keep bad stuff out of your gold plating bath.
I am not a hands-on gold plater, just a bookworm, so I'm happy to be corrected if others say you should be able to do thick decorative plating from this strike bath ... but I will be surprised if I learn that :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
? Hi Elias,
What have you done to solve this problem.
-- Thanks, Jeevi.
Sales Engineer - India
February 2, 2025
![]() Thanks for trying to engage with Elias; we'll advise him of your posting and see if a reply results.
Thanks for trying to engage with Elias; we'll advise him of your posting and see if a reply results.
But place introduce yourself and your situation. Are you having the same problem of trying to generate thick decorative gold plating from a strike bath? Or maybe you have non-commercial potential solutions in mind? Your posting is too cryptic for anyone other than Elias to engage with you :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hi We are using Neutral bath at Au 7 g/l. We are using brush plating to achieve thickness 1.30 in connector part in reel to reel plating method. We got production 6 months no problem but now suddenly reddish appearance problem happenws.
We have checked Inorganic Impurities
Like Cu-0.4 ppm, Fe-22 ppm, Ni-32 ppm.
And did Activated carbon treatment.
Then did 10% dilution.
Increased rinsing time.
We check with low current density and high current density both have Red problem.
If we use cotton but to remove it can remove.
And we check the Clean dry air and changed filter.
But still we can't find the root cause.
Please advise on this.
Thanks
Jeevi
- India
February 5, 2025
Electrochemical Metallizing by Marv Rubinstein

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Jeevi,
Despite your analyses it certainly sounds like a contamination problem.
I have never seen an application of brush plating on a reel to reel wire plating line, so I'm at a bit of a loss. I don't know what the brushes look like, how you get the solution to them, how often you replace those brushes, etc. Even Rubinstein's "Electrochemical Metallizing" doesn't mention brush plating of wire (or anything else in a continuous reel-to-reel line).
About all I can suggest is testing with a Hull Cell
⇦ on
eBay
or
Amazon [affil link] to determine the effect of current density, and to learn if something about the brush arrangement, rather than the solution, is causing the problem.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I am using CN-based^Alkaline non-cyanide Gold bath pH level 12.5
Operating temperature : 80 degrees
Initially my plating bath solution is very clear & transparent; now it's the 3rd month and I have plated about 40,000 sq. inches. Now it turns from clear to dense colour and I facing reddish plating finish now. How to clear impurities, and what measures I have to take? Please suggest. It's a gold flash bath and I am doing decorative plating bronze metal, then bright nickel, then gold flash, then spray lacquer coat.
Now in my bath pH is 11.5. I used to give power supply through copper wire to plating component so I feel red shade is due to copper contamination. If I confirm it is copper contamination by lab analysis how should I remove copper contamination? What steps do I have to take to maintain it clean without impurities in my bath?
A. Vasanth karunakaranEmployee - Chennai, India
October 21, 2021
Q. Sorry -- only now I found out it's an Alkaline bath not a cyanide bath.
A. Vasanth karunakaran [returning]Employee - Chennai, India
October 25, 2021
Q, A, or Comment on THIS thread -or- Start a NEW Thread