
-----
Brown Spots appear 1 week after nickel electro-plating on brass coils
2006
Hi folks,
I have some age-old problems with nickel electroplating on Brass coil.
After send in for plating, there are brownish/ yellowish spots erupting from the surface.
I wonder if it is plating or material problems?
The plater told me it's the pores of the brass coil and the sulfuric acid can't even clean away the impurities but "trapped" in the tiny grains of the material and start appearing as yellow spots...
Is that a feasible explanation?
I have seen black and white spots appearing on the coils 2 to 3 years ago but never see yellow spots before...
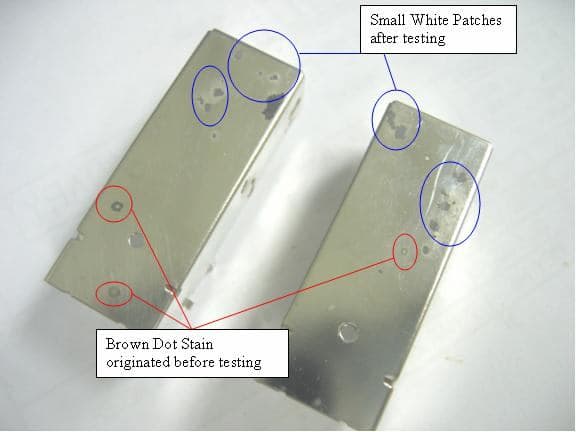
Hope some of you can assist me with this long pressing problem.
Yours sincerely,
Alan ChowCoil Slitting - Singapore
Hi Alan,
It is very possible what the plater is saying is correct, but it sounds like they could be using a better pre-dip than sulfuric acid or a sulfuric acid based pre-dip. There are proprietary peroxide based dips that offer different degrees of etching and brightness, and are easy to waste treat. They do a great job in cleaning the surface of the brass. What is the thickness of the nickel plate? Insufficient plating thickness could also be a cause of the problem. The plater should be investigating better cleaning techniques and try to solve the problem, rather than inferring "it's the nature of the beast". You could try sending a sample lot to a reputable plater, and let them know the problems you currently have. If they want your business they will do everything to get it right. Good Luck!
process engineer - Malone, New York
2006
2006
Dear Mark Baker,
Thanks for your advice & expertise.
Instead of using 1.27um Nickel, we are only adopting 0.5 µm Nickel undercoat before adding Bright Tin.
Is that the industry standards of using 1.27 µm for Nickel undercoat in order to cover colored spots?
We do not have black spots erupting , my old samples tat are 2 years back did not show any signs of spots erupting....
I need your advice... it may be coil material or cleaning agent?
Yours sincerely
Alan
Coil Center
- Singapore
You have simple galvanic corrosion setting up in the pits. YOu may retard this tendency by dipping in a weak solution of hex chromic acid. The rinse following a chromate or chrome plate will greatly reduce the tendency for these pits to bloom. The brown color is copper oxide diluted with the zinc and nickel salts in the area. Hot-cold-hot-cold rinsing before dipping in the chromic acid will further increase the protection.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

2006
Hello again Alan,
In your first posted question you had said "send in for plating". Does your company have it's own plating department or are you using a jobshop plater? The reason I ask is to determine how much control you actually have to rectify this problem. If you have your own plating shop, the responses given on this site can be implemented by you or your plating manager. As Mr. Probert and I have mentioned the problem stems from the substrate material (brass). To alter the types of plating and the thickness of the Ni, is like putting on a band-aid, hoping to stop the bleeding. The issue of pore corrosion has to be dealt with first. Reducing the Ni thickness to 0.5um is not a good idea regardless of what the final plate is. I would concentrate on the pre-plate cleaning cycle, before you change anything else. You've been given a few recommendations to begin with. Good luck!
Process Engineer - Syracuse, New York
2006
July 5, 2011
Hi Alan,
We are into exports of Solid Brass Urns from India. We are also facing same problem like yours i.e Brownish Spots after lacquering. They are appearing in almost every finish we are doing like Brushed Matte, Pewter and Gun Metal..
We tried several platers and also tried different materials to finish our products to reduce the occurrence of these spots/blemishes and reduce buyer claims but nothing has worked out so far... We have reached the conclusion that this is not a plating defect. It is actually due to tiny pores in the surface of Brass which absorbs certain chemicals and moisture during various procedures that also include welding of two different parts, etc. Therefore, in order to avoid the spots, we need to have some chemical in which we can dip or wash our products before finishing them to be sure that the absorbed chemical and impurities are washed away...
I request readers to suggest some solution for this problem...
- India
July 24, 2011
HI
The sheets need a chemical polishing rather than cleaning only. Polish it for 30 - 40 secs -followed by sulfuric dip and then plate.The stain will never occur

Vasudevan Narayanan
- Chennai, Tamil Nadu, India
Q, A, or Comment on THIS thread -or- Start a NEW Thread