
Brass is Pink not Yellow
-----A. I've followed this thread with interest, wanting to clean several antique Edwardian brass door handles and finger plates. They needed cleaning up, but through the grime came the pink. I tried rubbing a lot with Brasso which made a negligible difference. I couldn't get the chemicals Goran suggested, but tried pickling a H2O2 and white vinegar mix, to no avail.
In the end I went a bit nuclear, but it seems to have paid off. I remembered I had some Ferric Chloride
⇦ on
eBay or
Amazon [affil link] solution from years ago that I used to etch copper circuit boards. I poured some into a plastic container and then got a clean sponge and carefully soaked some up (wear chemical/plastic gloves
⇦ on
eBay or
Amazon [affil link] and eye protection
⇦ on
eBay or
Amazon [affil link] ) and gently rubbed around all the places that were pink. The copper dissolves very quickly using this method, but you end up with quite a black-looking piece of brass. I actually went over the whole piece in this way, etching out plenty of bits here and there. I panicked a bit that I'd ruined it, but then I used a metal polish called "Peek"
⇦ on
eBay or
Amazon [affil link] which is available in the UK on Amazon. It's a blue-coloured paste rather than the liquid of Brasso.


Well I have to say that Peek works a treat -- much better than Brasso. Took a fair amount of rubbing -- I initially used a non-scratch pan scourer to get the worst off, then the sponge side and then a softer cloth.
All the handles have come up a treat - no pink tinges and a deep yellow brass colour. The handles are quite old so have plenty of surface scratches which I won't polish out as I don't want them to look brand new, but I'm really pleased with the results.
Hobbyist - Fareham, UK
October 26, 2023
Tip: Readers are welcome to browse this site anonymously!
But its main purpose is to build worldwide camaraderie through sharing.
Those who believe in this might not engage with anonymous posters.
⇩ Related postings, oldest first ⇩
Q. Having improperly soaked dirty brass (originally lacquered) bed fittings in a toilet bowl cleaner (HCl), a reddish pink copper color predominates the originally golden yellow polished finish. This red/pink color can be reduced by (excessive!) rubbing with a Scotch Brite pad. Is there a simpler chemical treatment that can reduce the elbow grease?
Neville A. GordonFang Marketing Limited - Kingston, Jamaica
2005
A. Hi Neville. My limited experience has been that commercial copper polishes like Brasso ⇦ on eBay or Amazon [affil link] will change that raw look back to the mellow look.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. You have dissolved the zinc from the brass, leaving only copper. To get the brass colour back, you will need to polish off the copper; if it is not too bad, Brasso or Duraglit ⇦ on eBay or Amazon [affil link] will do it. Do not use an abrasive agent unless the colour is persistent; if you do have to use an abrasive, you will need to polish out the scratches you will leave in the brass. Cleaning brass is not as easy as many people think, and a lot of damage gets done by people using the wrong systems. I suggest you should use a professional cleaning agent such as Brasso, etc., and do not try to take short cuts with strong acids and alkalies.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Q. I have just cleaned years of black stuff from a very old small ornate brass article with lots of very hard to reach nooks and crannies. The use of chemicals has left a pinkish tinge in the rougher parts, which I have tried to polish off with Brasso but the polish can't get into the nooks and crannies. I've tried a toothbrush but this does not seem to work either. Can someone recommend how I can polish off the pink from this article, I am reluctant to use any wire wool, etc., because the article is so old and ornate.
Jennifer Howellhobbyist - Derby, Derbyshire, England
2005
Q. I am restoring some solid brass window catches and began by soaking them in white vinegar
⇦in bulk on
eBay
or
Amazon [affil link] & salt. That removed the dirty dark brown coating but they were still rather 'greasy' to the touch so I soaked them in warm, soapy water with a bit of ammonia
⇦ on
eBay or
Amazon [affil link] which removed the grease but left them a pink colour instead of yellow.
I have spent hours googling this issue looking for a chemical solution rather than manual removal. As on this thread, many people suggest using Brasso to get the pink back to yellow but it's not having any effect on these catches that are pink so I'm still looking for the right method to get them back to yellow from pink.
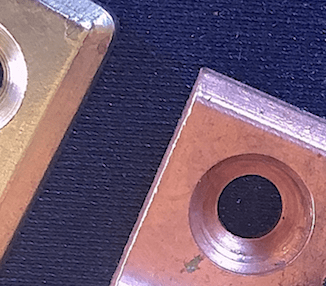

Any suggestions would be very welcome. Fortunately I only soaked a few of them in the ammonia and the others are still 'brownish' but these ones do come up yellow using Brasso.
Liz Gamlen- Waipukurau, Hawke's Bay, New Zealand
June 20, 2017
A. Brasso is okay. Try 5% ammonium citrate solution, pH 9 (50 gms citric acid ⇦ on eBay or Amazon [affil link] , 1 lit water, add ammonia ⇦ on eBay or Amazon [affil link] until pH is 9). Good luck!
Goran Budija- Zagreb,Croatia
![]() Thank you very, very much, Budija Goran!
Thank you very, very much, Budija Goran!
I have just removed some of the brass hardware from the 5% solution and it is is beautiful yellow again. I buffed them with ScotchBrite ⇦ on eBay or Amazon [affil link] and Brasso ⇦ on eBay or Amazon [affil link] and they look as good as new. They only needed a few minutes in the 5% ammonium citrate solution. So easy!
Liz Gamlen [returning]- Waipukurau, Hawke's Bay, New Zealand
Q. I know most people want the brass to return to yellow after etching or pickling but I've etched some brass and really like the pink colours. Will they stay if I leave it and decide not to clean it back to brass colour?
J monte- Barrie Ontario
March 24, 2023
A. Copper coating on your brass objects is very thin (you removed zinc from surface of it ), clear coat can help a little bit but best solution is copper plating. Hope it helps and good luck!
Goran Budija- Cerovski vrh Croatia
March 27, 2023
Q. I need assistance.. PLEASE!
I purchased a beautiful 1980s Louis Vuitton Toiletry case, and the person whom I purchased this from, did something to the brass zipper.. it is PINK! She won't tell me what she did. I have cleaned so many of these products with Brasso and they shine so goldenly.
She said, she used Brasso, but I don't quite buy it. Perhaps she left the Brasso on too long? Can I restore the pink zippers back to gold? Please! I am ready to get my science experiment on! Thank you all in advance for your help.
- New York, New York USA
March 15, 2018
![]() Hi Maria. Please see Liz's problem, Goran's suggestion, and Liz's rave review of how well it worked and so easily. I don't think anyone will be able to improve on it for you; but please get back to us after you try it. Best of luck.
Hi Maria. Please see Liz's problem, Goran's suggestion, and Liz's rave review of how well it worked and so easily. I don't think anyone will be able to improve on it for you; but please get back to us after you try it. Best of luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
|
|
A. Harpic Power Plus 10X is better than bleach in a black bottle. - Deganwy, Conwy, Wales, UK September 3, 2021 A. We are grateful for all participation, and Harpic probably is a good disinfectant, but I strongly doubt that it will turn brass from pink to yellow. Nobody mentioned bleach so, apologies, but I don't know what you are saying Harpic should be used for or why :-( Regards,  Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted is available for instant help or longer-term assistance. |
Q. OK, I have never done this before.
How do I mix that solution? Where do I get the products? I know, might be silly. I don't want to blow my fingers up! :)
- New York, NY USA
March 15, 2018
A. Hi Maria. We included links to where you can get the citric acid and household ammonia that Goran mentioned, and pH paper. But the internet, like a library, is a one-room schoolhouse where people of all experience levels can read about mixes & procedures they may or may not be qualified to follow. Maybe you can find a friend with some chemistry experience? >Safely working with chemicals is something you should be taught hands-on.
Although citric acid and household ammonia don't sound especially dangerous to me, nobody can explain to a person whose experience level they don't know, how to safely buy, store, handle, identify, and mix chemicals they have no understanding of. But you surely must not do so without wearing goggles
⇦ on
eBay or
Amazon [affil link] and rubber gloves
⇦ on
eBay or
Amazon [affil link] , and with good outdoor ventilation, and unless you've had at least high school chemistry, understand how to follow Goran's advice of how to adjust the pH to 9, and your work area is free of pets & small children who could get into them, and unless you've read all warnings on the containers :-)
Good luck and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Is citric acid the same thing as ammonium nitrate?
Q. Is citric acid the same thing as ammonium nitrate? I'm trying to turn my brass hardware from the pink tint back to the yellowish gold.
Mila Stevens [returning]- Montgomery, alabama
September 26, 2018
A. No, Mila, ammonium nitrate is certainly not citric acid. Please have a friend with chemistry experience read Budija's instructions carefully and help you with this. And also please carefully read the warnings to Maria L about messing with chemicals if you're not a chemist. Even household chemicals are dangerous :-)
Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Ted, so you're saying citric acid and household ammonia will work as well as ammonium nitrate for taking the pink tint from brass?
Mila Stevens [returning]- Montgomery, Alabama
September 26, 2018
A. Hi again Mila. Nobody on this page said that ammonium nitrate will take the pink tint from brass? ... but perhaps someone somewhere did? Sorry, I don't know what you're referring to :-)
Liz asked how to remove the pink tint, Goran said to make ammonium citrate by mixing ammonia with citric acid, and Liz said is worked great. That's all I can say about the subject. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I am having trouble with the brass feet of a clock I'm restoring. At the suggestions of a clock restorer I cleaned the feet then soaked them in a 5% ammonium citrate solution with a ph of 9. The feet came quite clean but turned a pink copper color. (The lighter pressed brass columns and lions heads came out perfect) The next suggestion was to Brasso the feet with 0000 steel wool
⇦ on
eBay or
Amazon [affil link] . That only made the pink worse and seemed to leave the feet with a copper plate in some spots and raw black metal in others.
The next rabbit hole I went down was to pickle the feet in a 3 part hydrogen peroxide to 1 part white vinegar solution. This quickly dissolved most of the copper plate but started to heavily etch the metal and leave it black with much of the detail etched off.


So now I know the feet are ruined and that doesn't bother me. I'm just very curious if there's a way to bring back the brass color from this point in the process.
Austin KeyClock restorer - Adams center, New York USA
December 1, 2018
A. Hi Austin. I'm on record on this site a dozen times discouraging people from trying to guess what a finish is from its appearance ...
Nonetheless, I'm gonna go out on a limb and say those feet might be cast iron rather than brass? Have you tried a magnet on them?
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Yes. I checked that first thing. And then checked again. I kept going back to that same thought, these must be plated. They are non-ferrous.
The paper I read by a chemist who also happens to be jewelry maker described the 3:1 H2O2:vinegar pickle. Another clock restorer referenced this article and his success with the process. The only part they tried that I didn't was a final rinse and scrub in Sparex pickling compound
⇦ on
eBay
or
Amazon [affil link] or 5% sulfuric acid.
Clock restorer - Adams Center, New York
A. Dear Austin!
Your object is probably copper plated zinc or tin alloy. Ammonium citrate at pH 9 solution is perfect for real brass, not for copper or brass plated zinc or tin. In that case better solution is 47,5 gms sodium gluconate
⇦ on
eBay
or
Amazon [affil link] / 47,6 gms citric acid
⇦ on
eBay
or
Amazon [affil link] and 4,9 gms tartaric acid
⇦ on
eBay
or
Amazon [affil link] / 1 lit water (according to USPT 4,264,418). Hope it helps and good luck.
- Zagreb,Croatia
Q. I have tried to follow the instructions to soak brass door finger plates which have turned pink. The only pH detector I can buy locally are tablets for fish tanks. Using the right proportions of citric acid, water and 20% ammonia solution never budged past neutral pH. Eventually there separated out a greasy yellow substance which floated.
This had no effect whatsoever on the brass.
What have I done wrong. I tested the pH tablets with straight 20% and the solution changed to purple, indicating that the tablets were accurate
- Bundaberg Qld Australia
March 19, 2019
A. Hi Margaret. What did you do to make them turn pink in the first place? If they were already pink when you first saw them, is there a chance that there's a lacquer clearcoat on them? But order pH paper ⇦ on eBay or Amazon [affil link] by mail if necessary; trying to do chemistry without pH paper or meter is wrong, and who even knows what else is in those tablets that could be messing things up.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. thanks Ted.
They do have a lacquer coat and had turned pink under the coating. My painter restored some; I have about 14 doors in the house with brass knobs and finger plates on each side. I thought the plates were copper but he restored them to a beautiful brass using a polishing wheel and rouge.
I used lacquer thinner/remover
⇦ on
eBay
or
Amazon
[affil link] Flammable!
to remove the lacquer and and salt solution to remove the tarnish. Even using brass or purple metal polish does not remove all the pink.
The knobs are filthy but not pink and all the lacquer has been worn off.
As a side note I am reluctant to re-lacquer the finger plates as it is such a pain to remove but equally it is difficult to keep the tarnish off.
Do you have any advice for any of my problems?
I also have window latches of brass. Thanks.
Margaret
(Actually I just reread my bottle and it is 20 g/l which makes it 2% not 20%. I tried again with a smaller quantity and got the pH up but still no effect on pinkness, only some tarnish removal.
- Bundaberg Qld Australia
A. I removed copper color from the brass material through following process and it's fine for me.
1. vinegar
⇦in bulk on
eBay
or
Amazon [affil link] + 5% sulfuric acid
followed by the Lemishine
⇦ on
eBay
or
Amazon [affil link] plus dish washing liquid for the shine.
2. 5% sulfuric acid + 3% peroxide
⇦ on
eBay
or
Amazon [affil link] but due to oxidizing process the brass gets dull.
Technical Services - Oakville, Ontario, Canada
Q. I've inherited an ATMOS clock from the 50's. I've tested the brass surrounding the glass frame: it is solid, not plated brass. I've removed a lot of tarnish using some ignorant processes along with Weiman's brass cleaner/polish; ended up with an inconsistent finish-yellowish/brass speckles and streaks and pink in places. I tried Goran's solution to no avail. Now I have very clean brass but it's not looking great. I suspect the brass frame has a lacquer top layer in which some of it is present, some gone. What can I now do to make this piece beautiful brass again? How can I determine the presence of lacquer and can/should I remove it? Also, what is the best way to buff/polish this? Thanks very much, I appreciate your help.
EJ Petersen- Reno, Nevada USA
December 1, 2019
Q. I have a brass plate rudder from an antique boat. It has random pink spots, like acne or chicken pox throughout the rudder. Any tips on how to get the pink spots out and get a consistent color.
Richard Heausler- New Orleans, Louisiana
April 4, 2020
A. Hi Richard. Goran suggested 5% ammonium citrate to get rid of pink color, and Liz G. gave a rave review of how easy it was and how well it worked. I wouldn't hunt for alternatives til I tried it :-)
Read the safety warnings on the chemical containers and this whole page. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello, I cast my own alloys. I use a ratio of 95% brass to 5% aluminium and usually that gives me a beautiful golden metal that I cast into weird and wonderful things.
Recently my casts have been coming out with this pink finish which I've learnt is my fault as I've overheated my crucible and I've burnt off the zinc.
Can anyone please tell me how much zinc I need to add back into my metal to give me the golden finish that I was so accustomed to seeing?
Many thanks and kind regards,
Matthew
- Orpington, Kent, England
April 18, 2020
Want to turn yellow brass tray to pink colour
Q. Hi everyone. I want to turn a brass colour tray to a rose gold pink colour. I can't find how to after ages searching google. Can someone please help?
Alison Vlismas- Wynnum West Qld Australia
October 6, 2020
A. Hi Alison. There are a thousand and one formulations for brass, so we can't guarantee that your results will be quite exactly the same, but Liz G accidentally turned her brass pink, included pictures and exactly how she did it, so we appended your inquiry to that thread :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. I was trying to clean an antique brass telephone and I have never cleaned brass before. As there were a lot of small details I used a toothbrush to rub Brasso on it and it has now turned pink in one spot. Is there anyway I can restore it back to how it was before?
Sofia Eastveld- Dallas Texas
January 18, 2021
A. Hi Sofia. We appended your inquiry to a thread where Liz G encountered a similar problem, Goran gave her an answer, and Liz said "good as new" and "so easy". It should work for you too!
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Hello,
I am following this thread with interest hoping I can save a few brass pieces. I am trying to buy the ammonium citrate. The only thing I can find is ferrous ammonium citrate, is this the same thing? Also, upon buying this I should create a 5% solution with water? Should I be careful about what brass comes in contact with it, i.e., only the pink part or is it not problem if it gets on other areas?
Thanks for any input!
- Houston, Texas
February 1, 2021
A. You can't use ferrous ammonium citrate. Citric acid
⇦ on
eBay
or
Amazon [affil link] can be found in every supermarket, ammonia 25%
⇦ on
eBay
or
Amazon [affil link] only in chemicals shop, pH paper
⇦ on
eBay
or
Amazon [affil link] probably too. (50 gms citric acid, 1 lit water, add ammonia until pH is 9).
A typical treatment regimen is as follows:
Solution containing citric acid (50 g) and Thiourea
⇦ on
eBay
or
Amazon [affil link] (10 g) in water (1 L) can be used too;
place the object in the solution and leave it until it is clean. This can take from several minutes to several hours depending on the condition of the object;
fully immerse the object in the citric solution to prevent the development of 'tide lines' on the object as these are difficult to remove, requiring extensive polishing;
brush with a soft bristle brush, such as a toothbrush, to speed up the process;
after cleaning, wash the object thoroughly to remove all traces of acid. Immerse the object in baths of clean water (preferably distilled) or under running water ... If you want shiny surface a proprietary metal polish can be used.
Hope it helps and good luck!
- Zagreb Croatia
Q. Hi all. I stupidly thought I could easily clean an old brass chandelier (I assume it is brass because that is what the tag said). It is currently brown color with a little gold coming through. I took one of the parts of the chandelier and rubbed it with Brasso. It now is pink, silver, and goldish. Can I put it back to the original color or do I have to do all parts and ruin this beautiful piece. Thank you!
Dana Ruffing- Pittsburgh Pennsylvania
March 19, 2021
A. Hi Dana. Unfortunately it's hard to tell what something is actually made of by just looking at it. This chandelier could be solid brass, in which case you can carry on and read Goran's advice about turning brass back to standard color instead of pink. But it might be just brass plated, in which case the plating may be worn away (a magnet won't prove it's solid brass rather than brass plated zinc; but if it's magnetic, it's steel or cast iron, not solid brass). If it isn't actually old, and it's silver colored in part, it could be a brass-tinted lacquer over nickel plating.
Test it with a magnet and tell us what you find, try to clean the ruined piece with lacquer thinner ⇦ on eBay or Amazon [affil link] Flammable! (to remove the lacquer if any), and send pics to mooney@finishing.com of the ruined piece and the whole chandelier for posting here -- and maybe someone can help
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Thanks for the advice. None of the chandelier pieces are magnetic. I will get some of the thinner tomorrow and see what happens. I also need to get pictures to show.
Thanks for the advice. None of the chandelier pieces are magnetic. I will get some of the thinner tomorrow and see what happens. I also need to get pictures to show.
Thanks!
- Pittsburgh Pennsylvania
Q. Hi,
I've got a Bronze watch and it has gone pink! I believe it was due to leaving in an acid cleaning solution to remove the patina.
I've read through the thread and seen the solutions but my concern is that my watch has a brown ceramic bezel, lume on the bezel, a steel and glass case back as well.
I have no idea what would happen to these elements if dipped in the peroxide mix and am concerned about damaging them.
Does anyone have any thoughts as to the safety of submerging the watch into the peroxide mix and if that's not the right move, any other options as I'm desperate!
Thanks!
- London
August 1, 2021
A. Hi Jonathan. I'd say don't dip it, use Q-tips ⇦ on eBay or Amazon [affil link] and patience. If the Q-tip doesn't offer tight enough control at an edge, use little pieces of black electrician's tape as a maskant.
But I would not use peroxide, I would follow Goran Budija's advice.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. @Goran: How to I test the pH level. Thanks.
Rhonda Ramsay- Sault Ste. Marie, On, Canada
August 30, 2022
A. Hi Rhonda.
The easiest way is with pH paper which is inexpensive and is simply dipped into the solution and turns different colors depending on the pH. You can follow this link ⇨
and see offers for smaller quantities as well.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread
