
-----
Gold plating over BeCu
this text gets replaced with bannerText

Q. Gold Plating Contamination Issue
Dear all,
My name is vijayakumar. I am from India. We are doing nickel, gold and silver plating at our end. Past two years we have beeen facing center contact contamination issue. We could not find this issue in our process but lately it will create one at our customer end.
The following inputs are helpful to you for better understanding:
1. The base material of cc pin is beryllium copper.
2. We are doing gold over nickel. thickness is 3 to 4 microns Ni, 1.27 microns Gold.
3. It is happening after assembly condition (finished product).
4. Passivation connectors (STAINLESS STEEL) only are affected which means outer body or other any components of the connectors got passivation.
5. The passivated components base material is stainless steel, grade 303.
Kindly help us to overcome this issue.



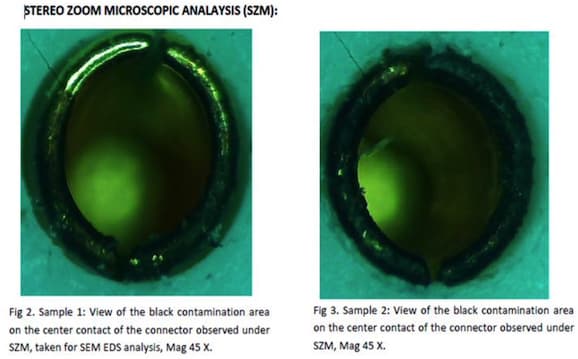
Picture is attached for your reference.
D. Dhanasekar, we also struck with same problem which you raised the issue on 2005. We want to know how you solved the issue or still the problem is in pending?
- CHENNAI TAMILNADU
July 20, 2023
A. Hi,
That ring around the gold plated contact (made of 303 SS?) looks badly attacked by the passivation chemicals. Have you considered citric acid passivation?
D. Dhanasekar's e-mail from 18 years ago was from the same company as yours :-)
This problem has apparently been very long-running.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
|
|
Q. Dear Sir, thanks for your input. Base material of gold plated component is beryllium copper and outer body made of SS303. it was done by passivation. Our requirement is treatment in nitric acid. - CHENNAI - TAMIL NADU , INDIA A. The gold is too thin and too porous to withstand the nitric acid passivation, go with Mooney on the citric. Also flash the be copper in acid copper first before nickel and gold - for better adhesion.  Robert H Probert Robert H Probert Technical Services Garner, North Carolina  |
A. Also remember that 303 is a free machining grade, meaning high sulfur content, meaning a "usual" passivation treatment used for 304 or 316 is not enough. "Rust" may in fact be sulfur rather than iron oxide. On the nitric side, the dichromate additive is usually done. With citric, a pretreatment in alkaline solution is called for.

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

July 25, 2023
A. Hello Vijayakumar,
I have had similar corrosion experiences with beryllium copper contacts plated with gold over nickel that have a requirement to pass salt spray testing. I agree with Mr. Probert regarding a copper flash prior to nickel plating. It is my experience that plating nickel directly on BeCu without a copper flash results in higher porosity of the nickel layer and in turn the gold above it. Increasing your gold plating thickness to 2.25 micron may also be sufficient to prevent this corrosion. Gold is expensive, but not as expensive as returns from the customer.
- Minneapolis, Minnesota
August 15, 2023
![]() Luke Soleim, Thanks for your input. Based on the experts inputs we took two samples respectively first batch we did copper under coat then in second batch we increased the hot water rinsing time from 10 minutes to 20 minutes. we kept the samples in our storage area more then 20 days and every day we have been monitoring the components through microscope. We did not notice any contamination on cc pin so far. Hence it is working.
Luke Soleim, Thanks for your input. Based on the experts inputs we took two samples respectively first batch we did copper under coat then in second batch we increased the hot water rinsing time from 10 minutes to 20 minutes. we kept the samples in our storage area more then 20 days and every day we have been monitoring the components through microscope. We did not notice any contamination on cc pin so far. Hence it is working.
Thanks for the below inputs:
Ted Mooney- That ring around the gold plated contact looks badly attacked by the passivation chemicals.
Robert H Probert - Also flash the be copper in acid copper first before nickel and gold - for better adhesion.
Ray Kremer - "Rust" may in fact be sulfur rather than iron oxide.
- CHENNAI TAMILNADU INDIA
August 17, 2023
⇩ Related postings, oldest first ⇩
by Reid & Goldie
-- hard to find & expensive; if you see a copy cheap, act fast!

on eBay or Amazon
or AbeBooks
(affil link)
Q. Hi,
We do Nickel and Gold plating over BeCu contact pins, which is used in the connectors.
The thickness of Nickel and Gold is 4 and 1.5 microns respectively. These pins are assembled with PTFE and finally assembled with SS parts (SS 303), which is passivated using Nitric and Dichromate.
After a few weeks the pins are found with brown contamination. Can anybody assist me to find out the cause and solution for the same?
D. DhanasekarElectronics - Chennai, Tamil Nadu and India
2005
A. Try to increase Ni.Thickness to 5 Microns.
Increase the hardness of gold.

T.K. Mohan
plating process supplier - Mumbai, India
Q. Thanks Mr. Mohan, We tried with more thickness, up to 6 microns and still the problem exists. Please advise suitably.
D. Dhanasekar [returning]Electronics - Chennai, Tamil Nadu and India
A. Mr Dhanasekar
Presumably the pins have a brown film where they contact the SS. If this is the case you need to rinse the SS pins in very hot Deionised water after passivating. 90 to 100 °C.
If the above does not apply, and the pins have a brown film on NON contact areas , after the plating cycle you need to rinse the plated parts in hot DI water. 90 to 100 °C. for 2 minutes. With some manual shaking and agitation. There is a possibility that cyanide remains in the pores.
The last point to check: Whether your Nickel solution could take a Hot Activated carbon treatment if it is contaminated. Activated carbon @ 3 gpl at 50 °C for 2-3 hours and filter thereafter. Hope this helps.
Regards,

Asif Nurie [deceased] [deceased]
- New Delhi, India
With deep sadness we acknowledge the passing of Asif on Jan 24, 2016
Q. [editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Electrical properties of BeCu. Hi there! Does anyone know will there be a dip in electrical property of BeCu once it is oxidized? Is gold plating necessary to maintain both the mechanical & electrical properties? Thanks!
quartz resonator manufacturer - Singapore
2005
Q, A, or Comment on THIS thread -or- Start a NEW Thread