-----
Black Spots/Patches in Acid Zinc Plating
Q. Black Patches in Acid Zinc Plating Reveal C, Mg, Ca & Si presence. From where these are expected and how can we avoid?
We electroplate plain carbon steel of 0.22% C in Acid Zinc. We are observing black spots on the plated product. We did carry out EDX of these black patches and we found that one sample is high in carbon content has traces of Calcium. Whereas the other samples has revealed Mg, Si, Oxygen & carbon content.
We would like to know:
1) What is this material from which these black patches are coming?
2) How can we avoid these black substances?
- Hyderabad, AP, INDIA
2004
A. Hello, Javed. EDX is a quite powerful tool, so it's great that you are able to bring it to bear, but it's rarely a good first step in troubleshooting plating problems. There's an old expression about how we "can't see the forest for the trees" and EDX can be a bit like trying to understand the forest better by looking at a tree with a magnifying glass.
Checking that operational parameters are within spec usually comes first, then "walking the line" and talking to the operators with a watchful eye towards anything unusual or that might have changed, followed by running a Hull Cell ⇦ huh? , will usually resolve the problem more directly.
My first guess of the cause of black patches would be iron contamination, and although that doesn't seem to align with the data you are seeing, dark patches at high current density on a Hull Cell panel would further suggest it. What I'd love to see while you're at it is an EDX on the Hull Cell panel so we can tie together the defects on a Hull Cell panel, which we pretty much understand on a macro level, with what you are seeing on a micro level :-)
Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
for Shops, Specifiers & Engineers

from eBay, AbeBooks, or Amazon

from eBay, AbeBooks, or Amazon
"Alkaline non-cyanide zinc plating with reuse of recovered chemicals" by Jacqueline M. Peden (1994)
from (U.S. EPA)

from eBay, AbeBooks, or Amazon

from eBay, or AbeBooks

from eBay, AbeBooks, or Amazon

from eBay, AbeBooks, or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
A. Javed Bhai,
A possible cause of black patches in Acid Zinc Plating has been listed by Ted to which I fully agree.
In addition:
- Look for re-cast zinc anodes that contain traces of lead or copper. These impurities deposit selectively in corners and crevices that are called Low Current density areas and show as black co deposits with zinc.
- Are the black patches "Burnt Zinc Deposits" which occur when the zinc metal is too low, or Total Chloride is way below the 125 - 140 optimum range, or in extreme cases when the solution temperature of operation falls below say 12 °C which is usually not the case in Hyderabad Deccan where woolens have just started to come out. !
OR it is simply lack of zinc deposit that shows up as black patches.
The cause is poor pre cleaning in this case.
Kindly analyse the problem from the above points of view first. Ensure the solution has adequate levels of basic chemicals, (Zinc, Total Chloride and Boric).
Ensure the additives are dosed correctly, not in excess or in short over rated levels. It helps to follow the appropriate Product Data sheet.
Regards
Sincerely.

Asif Nurie [deceased] [deceased]
- New Delhi, India
With deep sadness we acknowledge the passing of Asif on Jan 24, 2016
Q. Asif Bhai,
Shukriya!
What we are observing are black deposits in the recesses and threads of micro screws.
I am confident that these are not burnt spots. Is there any chance that these would have resulted due to excess brightener in bath?
Javed Pasha [returning]- Hyderabad, India
A. Mr Javeed,
Generally black spots occur in High Current Density Areas if the brightener is high in Acid Zinc plating. As Mr.Ted told the problem may be either iron contamination or Lead, copper contamination. If black spots are appearing after nitric acid dip in L.C.D areas then contamination may be copper.
Kindly check in Hull Cell.
N.K.PRAVEEN KUMAR- Secunderabad - India
A. Javed Bhai
If the threads are black (Kali Chudi) the possibilities are:
- Improper cleaning, overpickling, or simply dirt that hasn't been cleaned. You could check the parts just before the barrel is loaded to confirm this aspect.
- It may be co-deposits of lead or copper showing black after the first Nitric dip or passivate. If that is so, dummy till no problem.
- Are you adding sufficient M part with Brightener. In barrel operation use at least 50% M to R or go 1:1 if the suppliers data sheet says so.
At any cost avoid excess brightener. Read data sheet.
There is no substitute for good pretreatment. Check the cycle of cleaning personally.
Regards
Sincerely

Asif Nurie [deceased]
- New Delhi, India
With deep regret we sadly advise that Asif passed away on Jan 24, 2016
A. Hello Javed,
The reason for black spots is generally due to excess iron contamination. Sometimes in barrel operations if you are passing high c.d causes the same problem.if your bath is contaminated with iron it must be treated with 30% hydrogen peroxide.

Shoban Kesarapu
plating chemicals supplier - Secunderabad, A.P., India
September 1, 2008
Multiple threads merged: please forgive chronology errors and repetition 🙂
Black patches on casting after passivating with trivalent yellow
Q. After processing Sg iron casting in acid chloride bath with thickness about 20 microns ,we used trivalent yellow passivation, but after 30 to 40 jobs processed for the passivation ,black patches started arising itself in the passivation bath. Jobs processed initially are in good condition and appearance.Please help!
Rajendra Pawar- Pune, Maharashtra, India
2006
A. You can observe the black spots after the dip in the passivation but the cause is in the acidic zinc solution. This solution tends to dissolve iron and this iron can cause black spots in passivation.
The zinc plating solution should be treated regularly to eliminate the iron (you have to turn the divalent iron into a trivalent one by air or hydrogen peroxide and then to filter the sludge)

Sara Michaeli
Tel-Aviv-Yafo, Israel
A. Some additives are available which can remove and control the impurities.
If the bath volume is large, more than 500 Ltrs, you can reduce the impurities by Ion Exchange method.
Regards,
- Pune, Maharashtra, India
Q. You said the Acid chloride bath has the tendency to dissolve iron contents then why we have not faced such problem while using hexavalent yellow passivation?
Is the trivalent yellow passivations are fragile to handle?
- Pune, Maharashtra, India
A. Yes, the trivalent chromate is much more sensitive.

Sara Michaeli
Tel-Aviv-Yafo, Israel
Multiple threads merged: please forgive chronology errors and repetition 🙂
Trivalent Zinc on Cold Rolled Steel
Q. Hi Everbody!
2 questions - what are these black spots, and zinc tri substitutes?
I have a cold rolled wire which I'm plating in Yellow Zinc Dichromate. People are unhappy with its look and so I've moved to a Zinc Trivalent for a bright shiny look with the statement that it's going to have less
ASTM B117 salt spray performance.
Anyways, I'm seeing the formation of blackened spots after 24 hours. It does not look red. It does not look brown. They look not unlike burn spots. They look black. My boss says it's the result of the sacrifice of the plating. My colleague says its loss of plating. Somebody please help! What are we seeing here.
The Zinc Tri looks fantastic and I'd really like to give it the go ahead. Can somebody recommend any zinc tri alternatives which have the same bright look and cost about the same?
Thank you so much for your time and thoughts. This site is fantastic.
product designer - Chester, Connecticut, USA
2007
A. Hello, Nate; thanks for the kind words. Your general approach is probably fine. Bright acid zinc plating followed by a clear trivalent RoHS-compatible chromate should give you the bright look you seek.
But as for the spots, if the plating is unsatisfactory and you are not the plating shop, you usually must leave it to the plating shop to fix it or find another plating shop :-)
The readers are always eager to help, but the problem could be poor cleaning, or porosity and poor rinsing, or iron contamination; it could be so many other things, and troubleshooting a plating problem if we are not talking to the plater directly can involve so much back-&-forth and lost motion as to become impractical. But good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. DIN 50961 (JUNE 1987) PAGE 4, CHAPTER 10.2.1.2, WHICH IS A GERMAN STANDARD.
THEY SAY ABOUT BLACK SPOT IN PASSIVATIONS AFTER SALT SPRAY TEST IS NOTHING BUT IRON CONTAMINATION IN ZINC BATH IN CASE OF HEXAVALENT PASSIVATIONS WHICH RESULTS IN BLACK SPOTS AFTER SALT SPRAY; AND Fe304 in case of trivalent passivations -- so in both cases it is iron.
Maintain your bath properly, check iron and other impurities in ppm in any good lab available in your locality.
Maintain all parameters strictly by regular checking.
I am sure you will get desired results.
With best wishes,

Ajay Raina
Ludhiana, Punjab, India
![]() I will inquire with my plater about iron in the mix as suggested. I'll post again when I find out more.
I will inquire with my plater about iron in the mix as suggested. I'll post again when I find out more.
Thanks for your replies, this site is very informative and a goldmine to those who use it.
- Chester, Connecticut, USA
A. There is an excellent discussion of this phenomenon in the Sur/Fin 2007 Proceedings entitled "Visual Degradation of Zinc-Surfaces in Salt Spray Tests - Black Spots and White Haze" by Dr. Sc. Marc Mertens of Enthone in the Netherlands.

Tom Rochester
CTO - Jackson, Michigan, USA
Plating Systems & Technologies, Inc.

Q. Hi,
Been googling for that article on Visual Degradation. Could not find it and I would very much like to get my hands on it. Can anybody link me?
Thanks again for your help! I've had much success thanks to your collective expertise.
- Chester, Connecticut
A. Unfortunately, those conference proceedings are not something in the public domain that you would find on the web, Nate. You'd need to buy them on a CD-ROM at a cost of something like $325 from www.nasf.org.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 27, 2008
Multiple threads merged: please forgive chronology errors and repetition 🙂
Black Spots in Acid Zinc Plating at LCD after Trichrome Passivation
Q. Black spot shows at Lcd area in Acid Zinc Plating after Trichrome passivation. I want to Know What is the reason behind this black spot.
Sandip Patilfield officer- Mumbai, Maharashtra& India
September 27, 2008
|
|
A. Most likely iron contamination. Clean out your tank more often.  Trent Kaufman electroplater - Galva, Illinois A. Hi sir,  Shoban Kesarapu plating chemicals supplier - Secunderabad, A.P., India |
Black Spots in Zinc Trivalent Plating
Q. We are brake manufacturing company in India
We have a peculiar problem of black spots on zinc trivalent plating.
This is done on a brake item - caliper (rear brake)
The base metal is SG Iron - FE 410 grade,
casting is machined and sent to plating.
Initially we do not see this plating defect but after a day we see this defect.
Can some one from the team throw the possible causes for this defect and how it can be prevented?
Thanks,
buyer - Chennai, India
October 6, 2008
A. Dear Mr Vasudevan,
This problem can be minimised by de-gassing the part prior to plating; say for 20 minutes after the part reaches 150 °C. Also a soak in 50 g/l of Sodium Cyanide for 4 hours at 50 °C immediately prior to plating would also prevent the black spots from coming out.
Warm regards,

Khozem Vahaanwala
Saify Ind
Bengaluru, Karnataka, India

A. Mr. Vasudevan,
The main problem is due to poor cleaning, in the sense periodical oil level checking in cleaners and replacement of cleaners timely both soak and electro will dramatically improve this problem.
Also suggest to use better chemistry in plating and Trivalent passivates to overcome this problem.
Regards,

P. Gurumoorthi
electroplating process chemicals - Chennai, Tamilnadu, India
A. Dear Mr. Vasudevan,
1. Ensure proper degreasing and electrocleaning of casting components.
2. Try sulfuric acid pickling if you are currently using HCL.
3. Keep the metallic impurity level in passivation solution as per the manufacturers recommendation.
4. Ensure proper drying of components.
5. Try one lot with shot/sand blasting and observe whether the problem is repeated.
Hope this helps.
- Chennai, India
Q. Sir;
I'm facing a problem of black spot at upper side in bore in acid zinc rack plating bath.
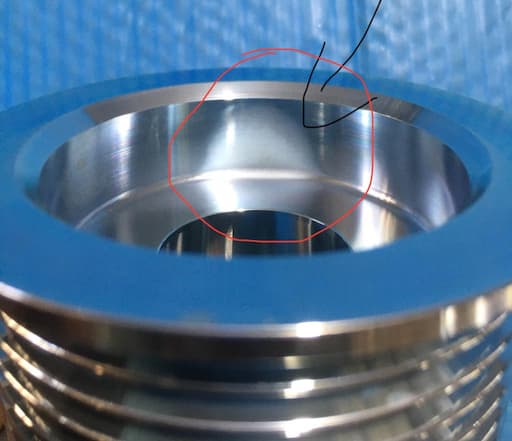

Process is below --
1)degreasing
2)acid cleaning
3)acid zinc plating
4)nitric dip
5)trivalent passivation
6)hot water rinse
7)hot air dryer
(2 rinse after all processes)
- Rajkot Gujarat
October 21, 2020
Q, A, or Comment on THIS thread -or- Start a NEW Thread