
-----
GALVANIC REACTION BETWEEN TIN AND ZINC CHROMATE
Hi,
I am a mechanical design engineer with my company responsible for specifying the materials and finishes on fasteners which are intended for products which are housed in outdoor telecom cabinets. Recently we have had some problems with the copper plating on our bolts and now we are considering making a change.
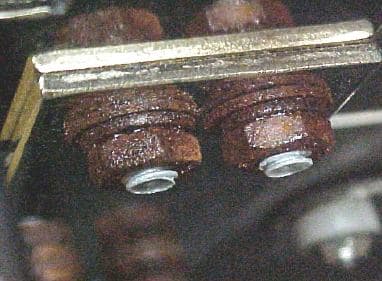
Typically the hardware (bolts, washers and nuts) secure 2 busbars together and the busbars are copper plated with tin. The PEMs in the busbars are steel with a zinc clear chromate finish whereas the bolts were CU plated but they have shown rust problems recently. My reaction initially was to go with the steel zinc yellow chromate fasteners, however the galvanic reaction between the zinc and the tin is concerning me. Yes I already have this situation with the PEMs but lets not make the galvanic reaction worst. Therefore despite the fact that the fasteners are yellow chromate coated should I still concern myself with the potential galvanic reaction between the zinc and the tin in my outdoor cabinet.
Giovanni SpinelliTELECOM POWER - Montreal, Quebec, Canada
2004
I'm sorry but I'm having a little difficulty understanding what we are looking at. I think I see badly rusted bolts, washers, and nuts. Do I understand that these were copper plated, but you think zinc plating will improve the situation?
It seems to me that tin plating the hardware, so it has the same potential as the tin-plated bus bar, would be the best way to minimize galvanic corrosion.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
2004
Q, A, or Comment on THIS thread -or- Start a NEW Thread