
-----
Wood's Nickel Strike Bath Analysis & Maintenance
Q. Hello everyone! I'm a chemist at a plating/anodizing shop in Utah. I've been carrying out bath analysis on our nickel strike bath and I have a question about the acid additions. In the literature, it details a desired HCl concentration of 10-11% v/v. I carry out the titration which gives me a value of fl oz/gal 36% HCl. Is the desired concentration value of pure acid or of 36% HCl? In other words, would I have to multiply my fl oz/gal titration value by 0.36 to determine just the pure acid concentration and go from there? Thank you all for your help!
Michael Warburton- Provo, Utah
April 14, 2021
A. Hi Michael
It is approximate percentage (volume by volume) from the concentrated HCl 36-37%, 22Be, Specific Gravity ~1.18. Be aware: some literature or manuals may refer to "original" concentrated HCl acid - as HCl 32%, 20Be, SG~1.16
Also, confusion is possible with Nickel Chloride in Woods strike: you can find recipes with concentration of Nickel ions only or concentration of Nickel Chloride (NiCl2) or concentration of Nickel Chloride Hexahydrate (NiCl2 x 6H2O)
Take a look here (nice article about strikes)
www.pfonline.com/articles/choosing-the-best-electrolytic-nickel-strike
Good luck
- Winnipeg, Canada
![]() Thanks for the help Leon!
Thanks for the help Leon!
- Provo, Utah
Q. Aloha,
How can you reduce or prevent the copper contamination from bus bars to nickel chloride solution?
- Nogales, Sonora, Mexico
December 21, 2022
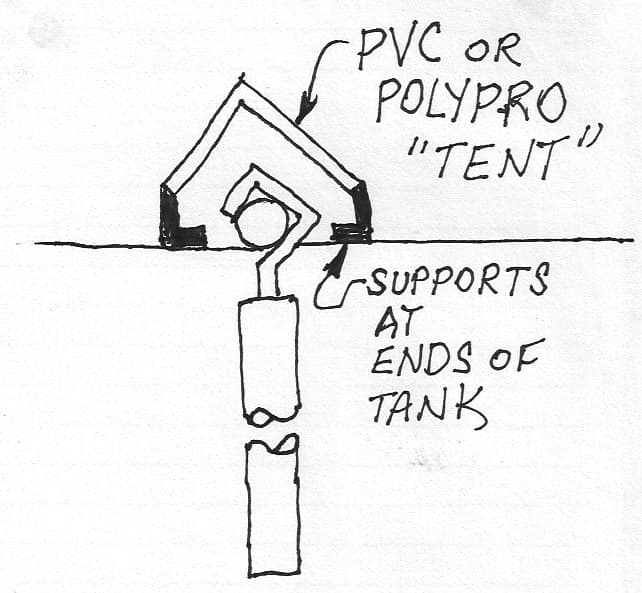
A. Hi Rodrigo.
Three alternate ways to limit copper contamination from drips onto anode bars --
• Plate the anode rods with something else ... electroless nickel is ideal.
• Put anode rod through a slightly larger soft plastic tube; make cut/slit in tube where anode hook makes contact with anode rod.
• Cover the anode rods with plastic drip shield "tents".
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() Thank you for your answer Mr. Mooney and Aloha!
Thank you for your answer Mr. Mooney and Aloha!
I'll try to cover the contact of the anode bar.
Happy new year 2023.
- Nogales, Sonora, Mexico
A. Hi Rodrigo
Many companies sell Titanium Clad Copper bars. Such bus bars have 1-2mm of Titanium around the Copper and it works well above many corrosive plating solutions.
Good luck,
- Winnipeg, CANADA
⇩ Related postings, oldest first ⇩
One Wood's Nickel strike tank plates, the other doesn't
Q. One Wood's Nickel strike tank plates, the other doesn't
Hi,
I have a really puzzling problem. We recently set up a small Wood's Nickel strike bath. The size of the new bath is 106 liters, 30 x 70.5 x 60 cm deep. We were using a large bath 622 liters, 75.5x 90.5 x 101 cm deep. Both of the baths have the exact same concentration of nickel chloride (70 g/L) and hydrochloric acid (13 g/L). We are able to get plating in the large bath but are not able to get plating in the small bath using the same current density. The customer specifies that we use a maximum of 2.5 volts so we cannot increase the voltage. Can anyone tell me what I would need to do to plate in the small bath?
Thank you for your help. Best regards.
Aysun ScottMetal Finishing - Kansas City, Missouri, USA
2003
A. If the solutions truly are the same, you should be able to prove it by plating Hull Cell ⇦ huh? panels from each; that would be the first step. Once it is established that the solution is not the problem, you may need to look at the geometry of the installation. 2.5 volts leaves very little for solution resistance and it could be that the shape of the parts precludes the operation from working at 2.5 volts. The resistance of your Wood's nickel bath is probably in the vicinity of 5 to 10 ohm-cm and you may find that the anode-cathode distance needs to be impossibly small to plate at 2-1/2 volts.
Trying running a calculation, because our intuition can be quite wrong depending on the specifics of the parts that are being plated. I was involved in an installation once where 18 volts wasn't enough for bright nickel plating--which we didn't find out until the line was built because it simply never occurred to any of a large team of highly experienced platers and consultants that it could possibly be a problem and nobody ran the solution resistance calculations :-(

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Considering that your small tank should have a closer anode to cathode spacing, I would have expected it to have done better. Based on that thought, I think that you have a line loss or connection loss. If it were mine, I would be checking voltage and amperage at every spot possible , right up to the parts and the anode. I would guess that your instruments are calibrated? Another thought is that you piggybacked it after the large strike tank and you are having some kind of losses to that tank. 2.5 volts is a really small voltage for a Wood's tank unless the part is tiny.
Tell us some more of your setup and what you have checked. This is interesting.
James Watts- Navarre, Florida
A. 70 gr/l of nickel chloride and 13 of HCl seems too low to me. I would give it a try with a higher concentration of both (up to the more common 250 gr/l each). This will increase the conductivity of your solution and improve the operation at low voltages.
Guillermo MarrufoMonterrey, NL, Mexico
Q. Thank you for your responses. We did check voltage and current. Isolated the anodes. Used different power supplies even replaced the anodes thinking the anodes may be passivated. We even tried dummying tank at low current to get rid of potential contaminants. We did not do a Hull Cell. We are in the process of doing a Hull Cell to compare the two tanks. In the meantime, we suspected that the wrong acid might have been accidentally added to the tank so sent a sample to the lab for analysis. It turns out we have .06% sulphates in the tank. Sulfuric acid and hydrochloric acid come in identical containers so someone probably accidentally picked up the wrong container. Will sulphates cause the bath not to plate?
Aysun Scott [returning]- Kansas City, Missouri, USA
![]() The problem is solved. Made up a new bath and the parts plate OK. It had to be the sulphates in the bath. Thank you all for your help.
The problem is solved. Made up a new bath and the parts plate OK. It had to be the sulphates in the bath. Thank you all for your help.
Best regards,
Aysun Scott [returning]Plating Operations - Kansas City, Missouri
A. A simple test for sulphates is to get a beaker of bath and mix in a little Barium Carbonate. sulphate will precipitate out. If you have a Chrome plating operation your lab probably has some Barium carbonate around along with an old Kocour Graduated centrifuge to measure its concentration.

Dave Kinghorn
Chemical Engineer
SUNNYvale, California
![]() Hello Guillermo,
Hello Guillermo,
Your concentration is right, my concentration that I'm using is Nickel Chloride 30-34 oz/gal (263 g/L) and Hydrochloric Acid is 10-17 %. This concentration is about the same as yours and is working fine for me.
We are in touch.
Best Regards,
- Phoenix, Arizona
March 2, 2023
? Why do you run it so high? What type of base metal are you trying to plate?
I have seen recipes this high, but our suppliers or plating consultants otherwise has always suggested we use somewhere between 4 and 10 oz/gallon nickel metal.
We have had great success with this recipe. It has excellent throwing power (for a nickel strike) and is designed to activate high alloy stainless steels, inconels, and high phos EN.
I am interested to learn more about the high nickel metal strike and its applications. Again, it is a suggested chemistry makeup in many different literatures, I just have not met anyone who has used that yet.
Kindest regards,
- Hammond Louisiana
June 23, 2023
A. Hi. I don't know if Donald Wood published the suggested concentrations for his strike or not. But I'd say the "right" concentrations are 240 g/l of nickel chloride and 120 ml/l of HCl -- my reason being that those are the concentrations Jack Dini used for the "Adhesion" chapter of his Electrodeposition
[on
Amazon
or
on AbeBooks affil links] ... and contrary to the rest of us who hold opinions on concentration, ASF, and whether to start with an anodic cycle and/or sulfuric acid, Jack published results -- including an astounding 81,000 psi ring shear strength :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() That is why I am asking. I have seen the recipe for it, but have not met anyone who uses that concentration.
That is why I am asking. I have seen the recipe for it, but have not met anyone who uses that concentration.
I assume there is a specific family of alloys or part geometries this is best suited to. That is what I was looking for.
We always used a lower metal and higher acid for better throwing power in LCD areas and better activation on high alloy stainless.
Thank you for linking the book. I do not think I have ever seen that before!
- Hammond Louisiana
June 26, 2023
Multiple threads merged: please forgive chronology errors and repetition 🙂
Maintenance of Wood's Nickel Strike Bath
Q. Does anyone have any suggestions for maintenance control for a Wood's Nickel bath ?
Dickie MartinEngineering - Laurens, South Carolina
2007
A. If you are NOT using the tank everyday, remove the anodes and set them in a tank of water and cover the tank. If you are using the tank only once in a while, also remove the anode bars and store dry - also cover the tank.
When you go to use the tank, top up the tank to just slightly below the full mark to allow for the anode displacement, stir well and analyze 2-4 hours later. Make additions as required, stir well and analyze the tank 2-4 hours later.
Copper buss bars dripping into the tank from condensation is probably a major cause of tank pollution.
Nickel growth is probably the biggest problem after drag in that you will have with a Wood's tank. They last for years with occasional filtering and dilution.
- Navarre, Florida
A. The last time I was in your plant you used s-rounds in a Nickel Chloride Strike. That will work, for a few days, then because of the activity of that kind of anode (easy dissolving), you build metal rapidly and the inefficient "strike" becomes an efficient "plating" bath, now it will not activate that burned on fired nickel on your ceramic.
Start with bagged Rolled Depolarized Nickel bars, take out when not in use; when the metal climbs, use bagged carbon anodes until the metal comes down. Be aware that insoluble anodes cause emission of deadly chorine gas, so be careful.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

A. First, remove the anodes when the bath is not in use.
Second, do regular partial dumps of the solution to control nickel content. Because the anode efficiency of the Wood's Strike is greater than 100%, Ni builds up and there's really no way to stop it, that I know of.
Some oxidation reaction needs to happen at the anode surface, and the other ones that can occur are oxidation of chloride to chlorine (no fun to breathe) or oxidation of water to oxygen. Perhaps some one else knows of an inert anode that will only do the second ... I do not.

Dave Wichern
Consultant - The Bronx, New York
Q. We use Rolled oval de-polarized anodes in bags. We only have the anodes in the tank when using the bath. We only use DI water to maintain the tank level. I am interested in what parameters, tank analysis and tolerances should be used to maintain the bath. The bath make-up is 139 L Nickel Chloride Solution, 59 L 31.5% HCl & 218 L DI water.
Thanks,
- Laurens, South Carolina
Woods Nickel bath analysis
Q. Hi,
Can anyone suggest the bath analysis for Wood's Nickel bath for Nickel chloride as well as Hydrochloric acid content?
Thanks in advance.
Plating shop employee - Bangalore, India
November 27, 2009
by Terrance H Irvine

on eBay or Amazon
or AbeBooks
(affil link)
A. Any plating lab book will give you this. Analyze for nickel. Convert metallic nickel value to nickel chloride with the appropriate factor.
Analyze for total chloride. Subtract the chloride content from the nickel chloride value from the total chloride and you will get the HCl value. A simple way that should be satisfactory is to titrate for the acid with an organic indicator at about pH 3.5 and do it slowly with a dilute caustic so that you do not precipitate any of the nickel.
For a poor value, you can convert pH to HCl content.
- Navarre, Florida
Ed. note: Alex Sirota offers additional input on this further down the page.
Q. Hi.
I have a problem with Wood's Nickel Strike bath; my bath loses the capacity to plate. Some months ago we were working normally, we didn't plate very many components. Nowadays we plate more, and we've changed the solution two times in six months.
We changed the solution and we found some black particles in the bottom of the tank.
Someone can help me?
Or someone has had the same problem?
Thanks.
Universidad Tecnologica de Quer´taro - Santiago de Quer´taro, Mexico
October 27, 2017
A. Hi Gustavo. I suspect that a component fell off of the plating rack and was allowed to dissolve in the bottom of the tank, consuming your acid.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
![]() I'm going to analyze the particles to know if it was a component that fell off in the tank, or what is the reason.
I'm going to analyze the particles to know if it was a component that fell off in the tank, or what is the reason.
Thank you so much!
Universidad Tecnologica de Quer´taro - Santiago de Quer´taro, Mexico
A. The most common problem is that by leaving the anodes in the tank when not in use, the metal content increases. A strike is supposed to be an inefficient low metal high conductivity solution. If you leave the anodes in continually, it change to a high efficiency PLATING solution, opposite for activation, Make a new solution and take the anodes out when not in use. Also S-Rounds dissolve too fast for a strike, no, use Rolled Depolarized Oval Bars

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

Q. I will be obliged if anybody will give a procedure to estimate acid content of Ni-strike bath
Veerendra Singh Modi- Indore, M.P.India
May 21, 2014
Q. Does anyone mind sharing their titration methods for wood's nickel strike?
Glenn Johnson- Paso Robles California
January 29, 2021
A. Nickel analysis:
Take 2 ml sample. Add 100 ml of DI water. Add 30 ml 1:1 ammonia. Add few grains of murexide
[affil link] indicator to orange color. Titrate with 0.1M EDTA to a purple color.
Ni g/L = ml EDTA x 2.934.
HCl analysis:
Take 2 ml sample. Add 100 ml of DI water. Add few drops of bromocresol green
⇦ on
eBay &
Amazon [affil link]
indicator. Titrate slowly with 1.0N NaOH [1N NaOH on
Amazon [affil link]
to blue color for BCG indicator.
HCl (32%) mL/L= mL NaOH x 9.784.
- Lod, Israel
Q. Hi I'm a sating level tank, what would be the make up for a 77 gal tank for nickel strike? When I titrate what would be my metal, nickel and HCl acid operating concentration levels? Please help.
pedro galvez- paso robles california
February 9, 2021
A. Hi Pedro. The make-up of Wood's Nickel varies per different texts, but is very easy. I'd go with 2 pounds per gallon Nickel Chloride & a quart per gallon HCl. So, for a 77 gallon tank it's 154 pounds on NiCl2 and 19.25 gallons of HCl.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Thank you Ted M. I appreciate you answering so fast. And how can I titrate the nickel chloride level? Is there a recipe and what reagent can I use?
Pedro Galvez- Paso Robles California
A. Hi again Pedro. Sorry, I have no lab technician knowledge or experience, but Alex Sirota just finished telling your co-worker how to do it :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread