
-----
440C stainless steel heat treatment problem- surface effects
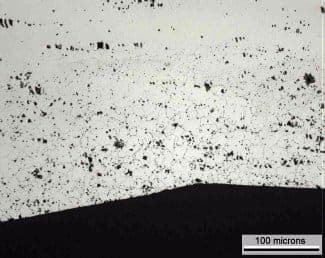
I am looking at a sample of heat treated 440C stainless steel that is soft at the surface (~ 30 HRC), and of normal hardness in the core (~50 HRC). The surface also exhibits dark grain boundaries in the as-polished condition (metallographic mount). Energy dispersive spectroscopy (EDS) of the polished grain boundaries shows elevated carbon. It is unclear if this represents chromium carbides at the grain boundaries, but I suspect not. I would imagine carbides at the grain boundaries would be white, not dark, and would not be continuous. The next possibility is intergranular oxidation, but I don't get a significant oxygen peak at the grain boundaries. There is a bit of silicon detected. The effect extends to approximately 150 microns in depth, and the grain boundaries are not severely thickened, but are quite obvious, as polished. This effect is observed on a ground part, and is seen on all exposed surfaces. I am wondering if the effect may have something to do with one of the following:
1) potential poisoning of the heat treating atmosphere with ammonia
⇦ on
eBay or
Amazon [affil link] , residual from an earlier furnace charge
2) residual cutting oil or other material on the surface

- Lansing, New York, US
2003
I think the evidence is in the low hardness , I would propose that the part has be decarburised by the heat treatment environment and possibly oxidised as well . Even though carbon is detected at the GB's this where carbides would migrate and are difficult to breakdown . But the bulk hardness of 440C comes from the martensitic background and so if the hardness is down at the surface so is the carbon .
Denis Gosney- London, England
December 8, 2008
Q, A, or Comment on THIS thread -or- Start a NEW Thread