
-----
Galvanic potential of aluminum after conversion coating
Q. ATTACHED IS THE GALVANIC SERIES OF METALS FROM MIL-STD-889. ALUMINUM IS A GROUP D-E METAL LISTED AS INCOMPATIBLE WITH EVERY METAL IN EVERY ENVIRONMENT (INDUSTRIAL, MARINE, SEA WATER).
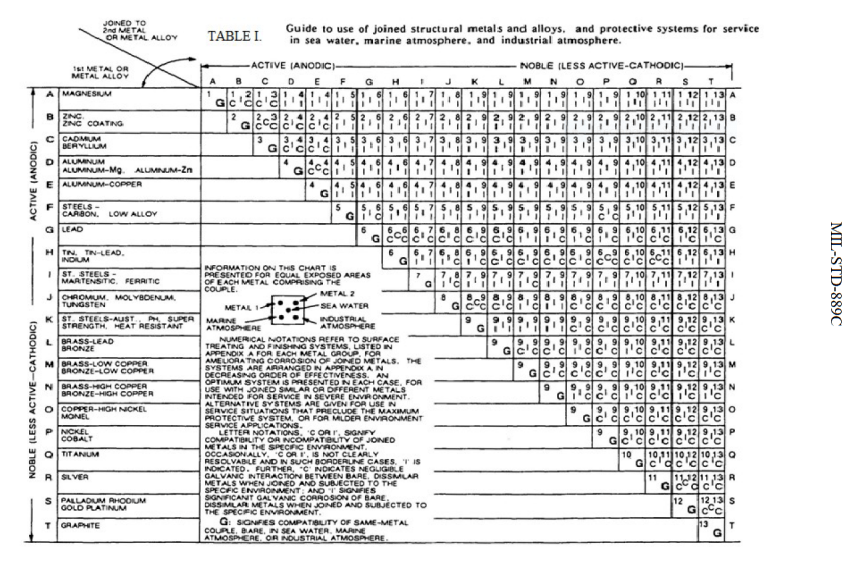
IN THIS APPLICATION WE WILL BE COATING THE ALUMINUM WITH A CHEM FILM/CHROMATE CONVERSION COATING PER MIL-DTL-5541
[⇦ this spec on DLA]. SO WITH THE CHEM FILM COATING IN WHAT GROUP WOULD THE ALUMINUM NOW BE IN THE GALVANIC SERIES OF METALS? GROUP J FOR CHROMIUM? FOR EXAMPLE IF THE ALUMINUM WAS PLATED WITH NICKEL IT WOULD BE GROUP P.
THANKS FOR ANY RESPONSES.
- GREENLAWN, New York
January 4, 2021
A. Hi Bill. Unfortunately chromate conversion coating will not achieve anything in this regard. We attached your inquiry to a thread where several people noted that MIL specs dictate no adjustment, and one reader even verified no change in galvanic potential from chromating.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
⇩ Related postings, oldest first ⇩
Q. I am the responsible mechanical engineer for my company's new naval electronics cabinet. I am evaluating the compatibilities of the materials proposed by our cabinet vendor. We have aluminum and steel in the cabinet. The steel is zinc plated with chromate conversion per ASTM B633 Type II (yellow). The aluminum is finished per MIL-C-5541. Where do the materials now fall on a galvanic compatibility chart? Do metals with chromate or other conversion remain at the same galvanic potential as the base metal?
Mark L. Whittum- Merrimack, New Hampshire
2003
A. The steel is covered with zinc plating, so you will be dealing with zinc vs. aluminum. This is a common construction method for computers and electronics used within an office or controlled environment and experience proves it isn't much of a problem under those conditions. But if it is to be used at sea I expect it might be a serious galvanic corrosion problem.
Any possibility of anodizing the aluminum? That would make it an insulator. Another possibility is to Ivadize or electroplate aluminum onto the steel in lieu of the zinc plating. Finally, cadmium plating is compatible with aluminum but is a cumulative toxin we are trying to get out of the environment.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. Thanks for the response! We have decided to do the following in order to reduce galvanic incompatibilities and reduce the corrosion potential:
1) Tin plate the steel instead of Zinc plate
2) Use Tin-plated EMI gaskets instead of Monel (not mentioned in the original email)
3) Anodize the aluminum components in the cabinet that will be in contact with the Tin plate
Your comments were helpful in that they confirmed our concerns regarding the Zinc plating and also highlighted the advantage of anodizing the aluminum.
We did some testing here that further clarified the galvanic compatibilities of zinc plated steel with chromate conversion. An electrical engineer here set up a crude battery using de-ionized water with able salt dissolved in it. Galvanic potentials between different materials were measured and the results are as follows:
DETAILS - TESTING
The difference in electric potential between the following materials when placed in salt water are as follows:
Copper (Cu) (MIL-F-14072 lists EMF of -0.20V)
Zinc plating (Zn) (MIL-F-14072 lists EMF of -1.05V)
Sample from vendor (unknown electric potential)
Chromium (Cr) (MIL-F-14072 lists EMF of -0.45V)
Test results (+ or - next to material indicates which pole of the battery they represented):
Cu+ to Zn- = +0.825V
Cu+ to sample- = +0.80V
Sample+ to Zn- = +0.054V
The vendor sample had the same galvanic potential to within 0.054V as the known zinc sample. The difference between the known copper and the known zinc matched the result expected from the galvanic chart to within 0.025V. FYI - the resolution of most galvanic charts is 0.05V and I assume the tolerance on these measurements is about the same. The chromate wash does not change the galvanic potential of the material surface. If it did the difference between the Cu and the sample would have been about +0.25V and the difference between the Zn and the sample would have been about -0.60V.
Conclusion: the test setup is valid and the vendor sample has the same galvanic potential as Zinc, presumably due to the zinc plating on the sample.
This result agrees with the recommendation from MIL-F-14072, "Finishes for Ground Based Electronic Equipment":
Para. 3.13.1 Use of compatible couples. The following should be considered in the selection and application of compatible couples:
a. Passivated coatings. For a compatible couple selection, passivated coatings specified herein shall be ignored and only the plating or basis metal considered. For example, all. chromate or phosphate treatments of zinc or cadmium specified in Tables III and IV shall be ignored in making couple selections and only zinc or cadmium considered as acting in galvanic corrosion. Hard anodic films on aluminum-base alloys are impervious nonconductors and, therefore, contact may be made with any dissimilar metal.
- Merrimack, New Hampshire
![]() Thank you very much for the followup!
Thank you very much for the followup!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Multiple threads merged: please forgive chronology errors and repetition 🙂
Galvanic potential of chromate conversion coating on aluminum in contact with gold
I have a printed circuit board (PCB) with gold plating over nickel. I have an aluminum housing that has to contact the PCB. That is a big galvanic couple. I have to keep the galvanic potential <0.1 volts.
What happens if I simply Alodine the aluminum in accordance with Mil-C-5541? Does that solve the galvanic incompatibility or not affect it at all?
- Allen, Texas
2007
A. Are you sure about < 0.1 volts? This even eliminates silver (-0.15V vs. gold); everything except platinum & rhodium.
MIL-F-14072D FINISHES FOR GROUND BASED ELECTRONIC EQUIPMENT doesn't allow consideration of any emf change due to chromate conversion coatings. See Paragraphs 3.12, 3.13 and Tables V, VI & VII. For sheltered exposures, Alodine® can be used for Al in contact with Al or galvanically similar metals (within 0.25 V). For dissimilar metals, plating must be used.
Although nickel plating has an emf difference a bit outside the limit (-0.3 V vs. gold), it may be the most practical option. In climate-controlled environments, up to 0.50 V difference can sometimes be tolerated.
If conductivity is not required, anodize the aluminum.
- Goleta, California
Rest in peace, Ken. Thank you for your hard work which the finishing world, and we at finishing.com, continue to benefit from.
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. Is it common practice to ignore chemical conversion coatings when looking at galvanic corrosion? I have a lot of chromate conversion coated aluminium parts and am not sure if I should treat it as aluminium or is there a special galvanic potential unique to the chromate conversion coating. Similarly, we have been looking at non chrome coatings like the Iridite NCP but aren't sure how this would change the metal interaction in comparison to chromate trivalent class 3.
Kosta Krontiris- Melbourne, Victoria, Australia
January 22, 2015
A. Kosta,
I suspect the main factor here is that only exposed metal can serve as the anode in a galvanic cell. If your part were only partially coated, then the galvanic corrosion would focus on whichever of the two, substrate or coating, has the lowest reduction potential (i.e., is more prone to undergoing oxidation). If the part is completely coated, the substrate is protected as long as that coating is intact, and only the reduction potential of the coating comes into play.

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

A. Hi Kosta. Mark Whittum has answered this question for us by referring us to a MIL spec which says to ignore the conversion coating; I'm personally not aware of any specs that say otherwise. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Reaction of aluminum conversion coating process on steel hardware (helicoils)
Q. Good Morning, my name is David and I work in a aerospace based machine shop as the quality supervisor. I have input responsibility on job planning, NADCAP processing and assembly sequence and methods. A most recent question has come up that I cannot fully answer myself and would like to know if others can help address this question.
I have an aluminum part which ultimately gets assembled with helicoils (both steel and drylubed) and steel dowel pins. I am contemplating installing the hardware prior to the conversion coating process but I am not certain if there are any consequences from the Alodine reacting with the steel, drylube or helicoils. If anyone can shed any light on this question it would be most appreciated.
QC Supervisor - Placentia, California USA
October 7, 2015
A. These should be installed after coating. Not necessarily because of the Alodine, but the pre-treatments which can very easily attack steel. Also, press fit dowel pins are not water tight, and very well may trap chemicals that could leach out after processing causing staining of the coating..

Marc Green
anodizer - Boise, Idaho
Corrosion of Zinc blue passivated screws assembled to sulfuric anodised Aluminium Component
Q. We manufacture & supply anodised aluminium component (Sheet metal) to European customer but from past some days we are getting complaint from customer that they are facing corrosion on the Zinc blue passivated screws assembled to anodised aluminium component after some time.
Note: These zinc passivated screws are passing 240 hours of salt spray.
Is corrosion happening due to screws in contact with the sulfuric anodised aluminium? Please advise.
Packaging will be Export quality.
Shipment is through ocean

Naveen Kumar
- Bangalore, Karnataka, India
July 5, 2016
A. Hi Naveen. We appended your inquiry to a thread where similar issues are discussed. I do not have a full answer, but I do have thoughts for your consideration ...
1. The passivation of the screws can be ignored in terms of galvanic compatibility -- so it's a zinc vs. aluminum galvanic couple. But if you can switch to zinc-nickel plating, that is more compatible with aluminum.
2. If the aluminum was anodized after fabrication, and the screws are machine screws, it would seem that there is no metal-to-metal contact, and thus no galvanic corrosion. If the screws are sheet metal screws, they cut into the aluminum, and there is metal-to-metal contact. But even still, if the whole panel is anodized there should be very little galvanic current available; that is, if the surface of the aluminum cannot corrode into solution because it is anodized, it will not steal electrons from the zinc plated screws and cause them to corrode.
3. It seems to me that either the particular screws in question are not able to survive 240 hours of salt spray, and there is something wrong with your sampling/testing plan which leaves you believing otherwise, or
4. The packaging is not doing what is intended and the assembly is being exposed to a very corrosive salt environment during transport.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
A. Naveen,
There are many possibilities but one idea is to salt spray test not just the screws but the assembly. Testing the screws by themselves does not prove much because when it is assembled a battery is formed and zillions of electrons will go straight to the screw and in the presence of an electrolyte such as salty air, mud, etc., the screw will corrode eventually. If you can greatly improve on the screw coating it might save the day. A bandaid would be vac packing the parts and backfill with dry N2 when shipping.

Blake Kneedler
Feather Hollow Eng. - Stockton, California
January 2, 2017
Q, A, or Comment on THIS thread -or- Start a NEW Thread