Learn to recognize problem areas, then swiftly institute corrective measures.
If you are plating with a chloride zinc solution, you are likely enjoying the benefits of cyanide elimination, improved bath efficiency, and exceptional brightness. As with any plating system, however, you are probably encountering one or more routine operational problems and taking them for granted.
The root causes of, and practical solutions for, five common problems associated with chloride zinc plating are addressed here. The problem areas are solution "growth," deterioration of titanium anode baskets, brightener oilout, color chromate adhesion, and treatment and removal of iron from the bath.
Due to the wetting agents in a chloride zinc bath, the plating solution will shed better than the rinsewater that's dragged in. This results in a growing volume of plating solution. To minimize or eliminate this problem:
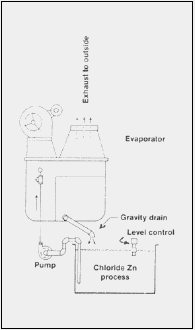
Fig. 1 - Atmospheric evaporator for controlling solution "growth."
(Courtesy Poly Products Corp., Atwood, CA)
Two additional benefits of evaporators are that they treat iron through aeration and they cool the bath to reduce or eliminate the need for cooling water or chilling.
In barrel lines, high voltage or an insufficient quantity of anode balls will result in the deterioration of titanium anode baskets.
Titanium forms an oxide film that prevents it from corroding in chloride zinc baths. The bipolar nature of this film permits current flow to the zinc anodes it contacts, but not directly to the solution. Voltages in excess of 8 to 9, however, will cause an attack of this passive oxide film and the basket will deteriorate rapidly, especially in the expanded mesh area. It is therefore important to keep the voltage low and the anode baskets full.
Titanium anode baskets with perforated polypropylene panels in place of expanded titanium mesh are available (Fig. 2). These are becoming quite popular because they practically eliminate the deterioration problem, even at elevated voltage.

Fig. 2-Titanium anode baskets with perforated polypropylene panels combat deterioration problem.
Brightener oilout occurs from excess additions of chloride salts to localized areas of the plating bath, thus supersaturating the solution and kicking out organics. Spreading these additions more evenly over the entire tank surface will mitigate this occurrence.
Other problem areas:
The ideal cycle following chloride zinc plating is as follows.
Many standard chromates work well. Several suppliers of brightener systems have also specifically designed chromates for chloride zinc with the intent of improving adhesion, color, etc. As with any type of zinc chromating, however, you may still be experiencing an adhesion problem from time to time, especially with iridescent yellow. This problem can be observed as parts come off the line or later, after storage. The routine causes and remedies are listed here.

Fig 3-Barrel welds should be checked for solution bleedout
To determine whether such a problem exists, leave the barrel rotating overnight in a rinse tank with 0.5 oz/gal black dye. Follow this with a rinse; trapped solution should show through the polypropylene. An alternative test is to spray methyl orange indicator on the barrel welds at the load stand and see if any red color change occurs. Bleedout solution is normally clear and acidic. Also, examine the barrels aHer weekend shutdowns for evidence of "salting" around the welds, indicative of the problem.
In rack plating, excessive iron buildup can cause yellowing. In barrel plating, iron codeposits as a result of the high current density that occurs the instant parts align themselves with the anodes through barrel perforations. Black spots of identical size as the perforations then appear on the work.
Iron buildup, a routine operational problem, can be minimized by exercising control of its three major sources, as follows:
Conventional treatment of iron with dilute hydrogen peroxide turns the bath red as the iron precipitates as colloidal ferrous hydroxide at pH 4.5 to 5.5. It is important to filter out this treated iron so it does not sludge on the tank bottom and coat the walls, cooling coils, and anode baskets. This occurrence is more apparent in barrel plating baths, where a great deal of iron accumulates from Sources 2 and 3 above.
Because excessive peroxide additions can increase the consumption of organics, air agitation has become an increasingly popular method of both naturally treating the iron and maintaining the iron hydroxide particles in suspension so that the filter picks them up. While air agitation has been used routinely in rack work, primarily to reduce burning, more recently it has been applied to barrel plating as well. A low-foaming bath should be employed.
As far as removing the red ferrous iron from the bath, the most efficient method revolves around the use of any media that can be precoated with diatomaceous earth. The media is not only economical to use, but absorbs the iron and screens it out. Filter bags, horizontal plates, and filter sleeves all work well as long as they can be precoated. The frequency of precoating is directly proportionate to the membrane surface area, so be sure to purchase a filter adequate in this regard.
In barrel plating, spotting can occur from untreated iron, but can also result from excessive high current-density burning. Both can be tempered or resolved by increasing the barrel rotation speed. Conventional rotation rates are 4 to 6 rpm. Rotation at 8 to 10 rpm will reduce the tendency to burn, so long as the type of part being plated makes this option feasible.
Chloride zinc plating offers considerable advantages vs. cyanide-based systems, although it is not without its share of routine operating problems. The remedies offered here are all used successfully by chloride zinc platers, and will greatly enhance the reliability of your operation.
 About the Author
About the AuthorStephen Schneider is president of John Schneider & Associates Inc., Mequon (Milwaukee), Wl. The firm has specialized in chloride zinc plating products and installations since 1967. Mr. Schneider holds a BS degree from the University of Colorado and is a member of the AESF Milwaukee Branch.
© finishing.com, inc., 1995-2025