world gathers for metal finishing
Q&As since 1989
-----
Energy efficient electroplating
Q. I provide technical assistance to industries and have been asked for information regarding energy efficiency in electroplating operations. What general information can be offered to experienced platers (zinc and chromate) regarding electrical connections other than to ensure good connections, have clean solutions, and operate within electrical non-peak demand times? This plater is aware of new equipment on the market, however, he wants to get the most out of what he has. Thanks for the assistance.
Ken BarnesState of Illinois - Champaign, Illinois, USA
1998
A. Hi Ken. For many years the AESF had an "Enercon" committee (I served on it) which did investigations and reports on the subject. One of the more exhaustive efforts was an AESF/DOE sponsored "Energy Conservation Study of the Surface Finishing Industry" by Mazzeo and Holcombe in 1978: AES Research Project 46. You should be able to get a copy of this from NASF, and hopefully some of the other materials. Although it's old, little has changed, so no need to reinvent the wheel.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
1998
Q. I am a graduate student working with my university performing energy audits of manufacturing facilities. Recently we went to an electrical conduit manufacturer, they make stainless steel conduit tubing. A very energy intensive portion of their manufacturing process is when they plate their stainless steel with zinc, which is why I'm posting onto this site. I was wondering if anyone had any suggestions or best practice suggestions that could be recommended to this company.
I have read a couple different postings on the site thus far and noticed that an efficiency improvement can be realized if the solution the product is dipped in is changed. The solution currently used is: zinc sulphate [on
eBay or
Amazon]
17-25%, Sodium sulphate 5.7%, Sulfuric Acid 0.2-0.4%, potassium thiocyanate
[affil links] 0.02%, and water as a balance. The company uses this solution to plate approximately 1 mm of zinc onto their conduit tubing. They have 14 rectifiers connected to a titanium based grid to make the plating possible. Each rectifier delivers around 13-16 V and 9800-13000 Amp, operation dependent upon condition of titanium grid and product quality. They currently run their product through this line during a 10 hour time frame for 2.5 days a week, is there possibility of decreasing the operating amperage to increase the time on the line and decrease the power demand of the system?
I would appreciate any insight that anyone has about what might be improved with this operation.
University of Kentucky - Lexington, Kentucky, USA
May 30, 2013
May 30, 2013
A. Hi Jake. The most essential ingredient in addressing the specific situation you asked about is Faraday's Law, and it is simplicity itself: if your rectifiers move a given number of electrons from the anode to the cathode (measured in Ampere-Hours), a given number of ions of zinc should be oxidized and dissolve into solution from the anode and be reduced from the solution as zinc metal onto the cathode. In the case of acid zinc, which oxidizes into the Zn+2 state, when you move 2 electrons, 1 zinc ion should follow. Faraday's Law says that 96,485 coulombs (ampere-seconds) will transfer 1 gram equivalent weight of metal if you are operating at 100% efficiency. If you look up the atomic weight of Zinc, and the density of zinc metal, you can also express this as 0.043 ounces of zinc should be deposited per ampere-hour, or that it will take 14.3 amp-hours to deposit 0.001" of zinc on one square foot of surface.
As mentioned, these numbers assume 100% efficiency (no electricity is wasted generating hydrogen gas from water instead of reducing zinc). So step one is to determine the present efficiency so you know the maximum theoretical energy savings possible. If the efficiency is approaching 100%, there is nothing that can be done to improve the deposition efficiency although it is very possible that replacing old rectifiers with new ones could deliver substantial energy savings.
Maybe start at .
This it is a well done, highly useful, one-page paper on the subject, linked to deeper data if needed. More universities & agencies than you can shake a stick at have gotten grants for "helping" the electroplating industry, so please start by downloading a couple of them rather than duplicating the spending and effort for the umpteenth time. Best of luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. Hi Jake.
Before I went any further, I would question why anyone would want to plate zinc onto stainless steel conduit.
Was it possibly a left over process from when the company once made conduit from mild steel?
My first reaction was that I could save a lot of energy by simply not doing it.
Please let us know if I misunderstood the question.

Geoff Smith
Hampshire, England
May 31, 2013
Q. Mr. Mooney,
Thank you for directing me to those articles by the University of Minnesota, they were very helpful in highlighting improvement opportunities. I noticed that they suggested using high-temperature potassium chloride and increasing the amperage to decrease the time that the product needs to be in the solution. The rectifiers that the company is using are operating around 14 V and 10,000 Amp with their zinc sulphate electrolyte. Would it be a good idea for them to switch to the potassium chloride solution? If so, what kind of resulting rectifier operation will occur?
Thanks,
Jake
University of Kentucky - Lexington, Kentucky, USA
June 5, 2013
A. Hi Jake. I didn't immediately see the info on high-temperature potassium chloride plating. If you give me a URL I'll be happy to comment. But the truth of the matter is that very few of the improvements suggested in such reports are still in effect even a few years later, and it's not because the shops are too lazy to bother continuing to save bags of money -- please be a bit skeptical about the savings proposed and their long-term practicality. The single most important element of energy efficiency in electroplating is to not generate rejects, and this is best done by keeping the process under control, relying on long experience and the suppliers' technical data sheets. Please calculate the present plating efficiency before proposing any process changes; the calculation is easy, not hard.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
June 5, 2013
Q. I want to decrease the energy consumption in my Nickel baths for electroplating. What can be done for such measures?
I have about 12 electroplating baths of Nickel in my vicinity, I wanted to decrease the energy consumption of every bath and make it similar in every one of them.
Which parameters should be worked upon to obtain electroplating on ideal basis to obtain best outcomes of energy conservation and improve productivity.
Abhi ParmarDesigner - India
February 10, 2018
A. Hi Abhi. We added your question to an existing thread which will help answer it. Download the University of Minnesota paper. Never change any operating parameter in a nickel plating bath in an attempt to improve energy efficiency. Instead make sure they are well controlled so there are no rejects -- there is nothing as energy-inefficient as producing rejected work. Improved energy-efficient lighting will also reduce rejects.
Things to look at first for improving energy efficiency include: covering the tanks when not in use with practical covers, switching to push-pull ventilation if you presently have pull-only ventilation, and replacing old energy-inefficient rectifiers with new energy-efficient ones if applicable. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
February 2018
Q. What do you mean by push pull ventilation in a bath?
I cannot add external air to the bath as it may hinder the process, increase the impurity, and decrease the desired temperature of the bath.
Also, I am having the practical hoods as well which are automated, my rectifiers are working at approximately 95% accuracy. Anything else can be done to achieve the desired?
Designer - Ahmedabad, Gujarat, India
February 14, 2018
February 2018
A. Hi Abhi. I have sketched a push-pull system to give an idea what I'm talking about ...
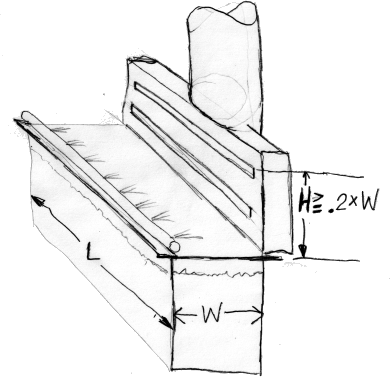
Industrial Ventilation
by ASGIH
on
AbeBooks
or
Amazon
(affil links)
and you can study the details of why it improves energy efficiency in "Industrial Ventilation" .
If you already have new highly efficient rectifiers, and you already have a good ventilation system which you are not going to change, and you don't have downtime during which you can make use of good tank covers, and you have good bright lighting so you instantly spot problems as they occur, you're done.
Sorry, but trying to improve the energy efficiency of a nickel plating tank by adjusting concentrations, operating temperature, and other parameters instead of following conventional practice and the supplier's technical data sheet would be a fool's errand. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread

